��Ŀ����
��ѧʵ��С�����۲ⶨ˫��ˮ��Һ�����ʵ�����������ʵ�鷽�����������ϣ������������ܶ�Ϊ1.42g/L�����ԭ������һ��������˫��ˮ��Ʒ��������̻�ϣ��ⶨ��Ӧ����������������
���̷�����
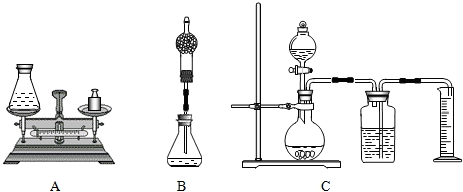
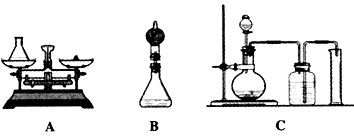
(1)�鳤���������Aװ�ã�������Ϊ75g����ƿ�м���0.5g�������̺�20g˫��ˮ��Һ������Ӧ��Ϻ�����ƿ�ͷ�Ӧ�������������Ϊ94.7g����д���÷�Ӧ�Ļ�ѧ����ʽ____��˫��ˮ��Һ�����ʵ���������Ϊ____��
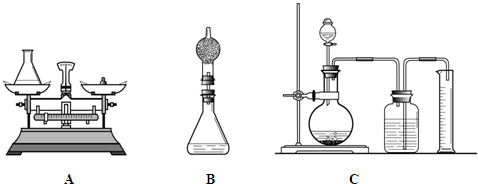
(2)С�ֶ��鳤�����������ɲ����Bװ�ý���ʵ�飨����ƿ�ϼ�һ����ܣ�����˵��С�����ɵ�������________��
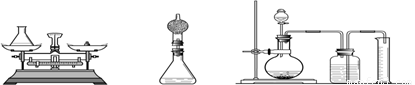
(3)С��ͨ��˼���������ǵķ������ϴ�����С�������Cװ�ã��Լ��������䣩��Ҫ����ʵ���Ҫ�������С����ѡ��____mL����Ͳ(��500mL��600mL��800mL)��
(1)�鳤���������Aװ�ã�������Ϊ75g����ƿ�м���0.5g�������̺�20g˫��ˮ��Һ������Ӧ��Ϻ�����ƿ�ͷ�Ӧ�������������Ϊ94.7g����д���÷�Ӧ�Ļ�ѧ����ʽ____��˫��ˮ��Һ�����ʵ���������Ϊ____��
(2)С�ֶ��鳤�����������ɲ����Bװ�ý���ʵ�飨����ƿ�ϼ�һ����ܣ�����˵��С�����ɵ�������________��
(3)С��ͨ��˼���������ǵķ������ϴ�����С�������Cװ�ã��Լ��������䣩��Ҫ����ʵ���Ҫ�������С����ѡ��____mL����Ͳ(��500mL��600mL��800mL)��
(1)2H2O2 2H2O+O2����8.5%
2H2O+O2����8.5%
(2)���������ˮ����
(3)600mL��Ͳ(��ʾ��Ϊ�������������Լ��563mL)��
 2H2O+O2����8.5%
2H2O+O2����8.5%(2)���������ˮ����
(3)600mL��Ͳ(��ʾ��Ϊ�������������Լ��563mL)��

��ϰ��ϵ�д�
�����Ŀ