��Ŀ����
��2010?��ɽ��һģ����ѧʵ��С�����۲ⶨ˫��ˮ��Һ�й������������������
[��������]�������������ܶ�Ϊ1.42g/L����ʯ�Ҹ�����������ƺ����������ƵĻ�����������ˮ�����Ͷ�����̼��
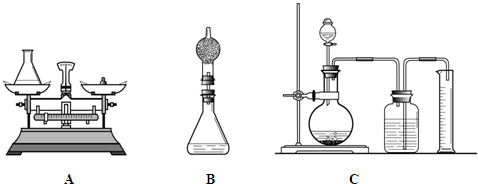
[���ԭ��]����һ��������˫��ˮ��Ʒ��������̻�ϣ��ⶨ��Ӧ��������������������������й���������������Ӷ���֪˫��ˮ����������������
[���̷���]��
���鳤������ƣ���ͼA������װ����Ʒ��Һ��20g������ƿ��75g����Ͷ��0.5g�������̣���˫��ˮ��Ӧ��Ϻ�����ƿ�ͷ�Ӧ�������������Ϊ95.1g�������������������
��С�ֶ��鳤��ͼA���������ɣ��������ͼB�ķ����������鳤��������ƿ�ϼ�һװ�м�ʯ�Ҹ���ܣ�Ȼ���ٲ�������˵��С�����ɵ�������
��С��ͨ��˼���������ǵķ������ϴ������������ͼC�ķ������Լ��������䣩����˵��С����Ϊ�������ϴ��ԭ��
[��������]�������������ܶ�Ϊ1.42g/L����ʯ�Ҹ�����������ƺ����������ƵĻ�����������ˮ�����Ͷ�����̼��

[���ԭ��]����һ��������˫��ˮ��Ʒ��������̻�ϣ��ⶨ��Ӧ��������������������������й���������������Ӷ���֪˫��ˮ����������������
[���̷���]��
���鳤������ƣ���ͼA������װ����Ʒ��Һ��20g������ƿ��75g����Ͷ��0.5g�������̣���˫��ˮ��Ӧ��Ϻ�����ƿ�ͷ�Ӧ�������������Ϊ95.1g�������������������
0.4
0.4
g����ʽ�����㣩��˫��ˮ��Һ����������0.0125
0.0125
mol��д�����������̣���˫��ˮ��Һ��������������Ϊ4.25%
4.25%
����С�ֶ��鳤��ͼA���������ɣ��������ͼB�ķ����������鳤��������ƿ�ϼ�һװ�м�ʯ�Ҹ���ܣ�Ȼ���ٲ�������˵��С�����ɵ�������
���������ˮ����
���������ˮ����
����С��ͨ��˼���������ǵķ������ϴ������������ͼC�ķ������Լ��������䣩����˵��С����Ϊ�������ϴ��ԭ��
������ƽ��ȷ�Ƚϵ�
������ƽ��ȷ�Ƚϵ�
�����Ҫ����ʵ��Ҫ���С��С����ѡ��500
500
mL����Ͳ����250mL��500mL��600mL�����������ٸ��������غ㶨�����������������ݷ�Ӧ�ķ���ʽ������������������������������������Ȼ�����������������ʵ�����˫��ˮ��Һ����������������
�ڸ��ݹ�������ֽ���������ʱ����߲���ˮ������
�۸��ݲ������������������ȷ����Ͳ��ȡ�ã�
�ڸ��ݹ�������ֽ���������ʱ����߲���ˮ������
�۸��ݲ������������������ȷ����Ͳ��ȡ�ã�
����⣺�ٸ������ⷴӦǰ���������������ٵ������������ɵ�����������������������Ϊ��������������������Ϊ20g+75g+0.5g-95.1g=0.4g��
��˫��ˮ�����ʵ�����Ϊx����
2H2O2
2H2O+O2��
68 32
x 0.4g
=
x=0.85g
˫��ˮ��Һ�������������ʵ���Ϊ��
=0.025mol
˫��ˮ��Һ��������������Ϊ��
��100%=4.25%
�ڸ�����ȡ������ҩƷ״̬��˫��ˮΪҺ̬������������Һ���ݳ�ʱ�����ˮ����������С�����ɵ������ǣ����������ˮ������
��������ƽ��ȷ����0.1g��ȷ�Ƚϵͣ�С����Ϊ�������ϴ��ԭ���ǣ�������ƽ��ȷ�Ƚϵͣ������������ѡ����Ͳ���������������Ϊ��
=0.28L��Ϊ280mL��Ӧѡ��500mL����Ͳ��
�ʴ�Ϊ����0.4g��0.025��4.25%�������������ˮ��������������ƽ��ȷ�Ƚϵͣ�500��
��˫��ˮ�����ʵ�����Ϊx����
2H2O2
| ||
68 32
x 0.4g
| 68 |
| 32 |
| x |
| 0.4g |
˫��ˮ��Һ�������������ʵ���Ϊ��
| 0.85g |
| 34g/moL |
˫��ˮ��Һ��������������Ϊ��
| 0.85g |
| 20g |
�ڸ�����ȡ������ҩƷ״̬��˫��ˮΪҺ̬������������Һ���ݳ�ʱ�����ˮ����������С�����ɵ������ǣ����������ˮ������
��������ƽ��ȷ����0.1g��ȷ�Ƚϵͣ�С����Ϊ�������ϴ��ԭ���ǣ�������ƽ��ȷ�Ƚϵͣ������������ѡ����Ͳ���������������Ϊ��
| 0.4g |
| 1.429g/L |
�ʴ�Ϊ����0.4g��0.025��4.25%�������������ˮ��������������ƽ��ȷ�Ƚϵͣ�500��
�����������ۺϿ�������������ȡ�Լ����ݻ�ѧ����ʽ���еļ��㣬��ɴ�����ĿӦ�þ߱��ۺϷ��������������

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ
