��Ŀ����
��ѧʵ��С�����۲ⶨ˫��ˮ��Һ�й�����������ʵ����������
[��������]�������������ܶ�Ϊ1.42 g/L����ʯ�Ҹ�����������ƺ����������ƵĻ�����������ˮ�����Ͷ�����̼��
A B C
[���ԭ��]����һ��������˫��ˮ��Ʒ��������̻�ϣ��ⶨ��Ӧ��������������������������й���������������Ӷ���֪˫��ˮ��Һ�й�����������ʵ�����
[���̷���]��
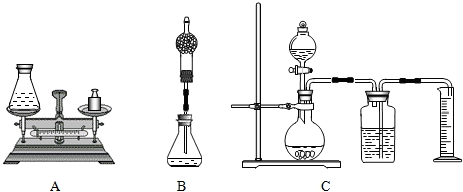
�鳤�������(��ͼA)����װ����Ʒ��Һ����ƿ��Ͷ��������̣���˫��ˮ��ȫ��Ӧ��Ϻ��ò���������������6.4g������ȡ����Ϊ mol��˫��ˮ��Һ���������� mol��д�����������̣�
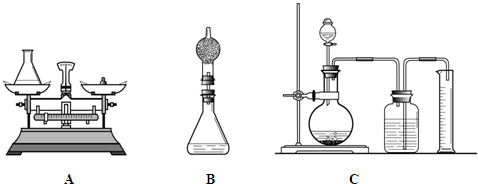
С�ֶ��鳤��ͼA���������ɣ��������ͼB�ķ����������鳤��������ƿ�ϼ�һװ�м�ʯ�Ҹ���ܣ�Ȼ���ٲ�������˵��С�����ɵ������� ��
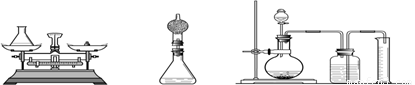
����C����ȡ�������������������������������Լ����
��1��0.4mol
��2�����������ˮ����
��3����Ͳ�е�Һ��������������Ͳ��Һ��������С����Ͳ��Һ������ �� 4.5��
��������
�����������1��������Ħ��������32g/mol������� n��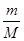 ��֪
��֪
6.4g���������ʵ���n��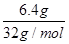 ��0.2mol
��0.2mol
��H2O2�����ʵ���ΪXmol������ݷ�Ӧ�ķ���ʽ��֪
2H2O2  2H2O
+O2��
2H2O
+O2��
2mol 1mol
Xmol 0.2mol
��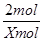 ��
��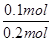
���X��0.4mol
��2���������������ˮ�������Ӷ��������ɵ���������ƫ��
��3������������ˮ������������Լ�ƿ�е�ˮѹ�뵽��Ͳ�У��������������������Ͳ�е�Һ��������������Ͳ��Һ��������С����Ͳ��Һ������������Ϊ�����������ܶ�Ϊ1.42 g/L���������������Ҳ���Ա�ʾΪV��6.4g��1.42 g/L��4.5L��
���㣺����˫��ˮ���ʵ���������������������ʵ����ⶨ��ʵ�鷽�����������

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�