题目内容
【题目】(1)已知某混合物由氧化铁、氧化亚铁和碳酸钙三种物质组成,其中铁元素的质量分数为28%,则碳酸钙的质量分数(用字母W表示)范围为____________
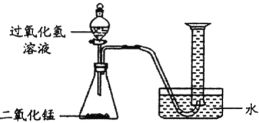
(探究)某同学为了测定10g该混合物中碳酸钙的质量分数,设计了如下图1的实验,请回答下列问题:(已知:浓硫酸有吸水性,可以作气体干燥剂)
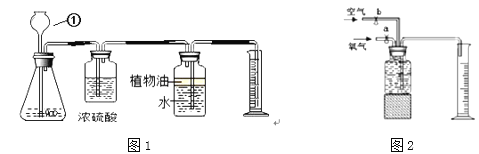
(2)仪器①的名称是_____________
(3)锥形瓶内装入的液体是稀盐酸,则生成气体的化学方程式____________________
(4)要得到准确的实验结果除了要知道进入量筒内液体的体积,还必须测量的数据是_______
(5)若实验结束后需要回收锥形瓶内的残留固体物,还需要进行的操作是________
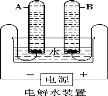
(6)某同学用如图2所示装置收集体积分数为60%的氧气(假设集气瓶中水被全部排入量筒中),则通入的氧气和空气的体积比大约是(整数比) ________
【答案】60%<W<64% 长颈漏斗 CaCO3+2HCl===CaCl2+H2O+CO2↑ 加入的稀盐酸体积 过滤 1:1
【解析】
(1)若矿石中含铁元素的物质全部是氧化铁,则矿石所含铁元素的质量分数为28%,则矿石中氧化铁的质量分数为28%÷(![]() ×100%)=40%;若矿石中含铁元素的物质全部是氧化亚铁,则矿石所含铁元素的质量分数为56%,则矿石中氧化亚铁的质量分数为56%÷(
×100%)=40%;若矿石中含铁元素的物质全部是氧化亚铁,则矿石所含铁元素的质量分数为56%,则矿石中氧化亚铁的质量分数为56%÷(![]() ×100%)=36%;由题意,氧化铁、氧化亚铁和碳酸钙三种物质构成的矿石,则该矿石中碳酸钙的合理质量分数在(1-40%)~(1-36%)之间,即该矿石中碳酸钙的合理质量分数在60% < W < 64%;
×100%)=36%;由题意,氧化铁、氧化亚铁和碳酸钙三种物质构成的矿石,则该矿石中碳酸钙的合理质量分数在(1-40%)~(1-36%)之间,即该矿石中碳酸钙的合理质量分数在60% < W < 64%;
(2)仪器①为长颈漏斗;
(3)碳酸钙与盐酸反应生成氯化钙、水和二氧化碳,化学方程式为:CaCO3+2HCl=CaCl2+ H2O+CO2↑;
(4)测定10g该混合物中碳酸钙的质量分数,需要计算碳酸钙的质量,需要通过计算二氧化碳的体积,二氧化碳体积=进入量筒内液体的体积-加入的稀盐酸体积,所以需要知道加入的稀盐酸体积;
(5)若实验结束后需要回收锥形瓶内的残留固体物,需要通过过滤将固体和液体分离;
(6)氧气约占空气总体积的五分之一;设通入氧气的体积为x,空气的体积为y;则[x+y×1/5]/(x+y)×100%═60% 解得x:y=1:1。

 心算口算巧算一课一练系列答案
心算口算巧算一课一练系列答案 应用题作业本系列答案
应用题作业本系列答案