��Ŀ����
����Ŀ��С����ʵ���о�������̼ͨ��ˮ�к����ı仯��
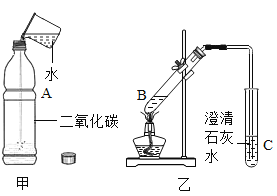
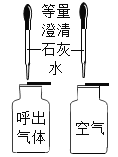
(1)��ͼ����ʾ����һ���ռ���CO2���������������ƿA�м���Լ1/3�����ˮ����������ƿ�ǣ�������ƿ�������_____(������������������)֤��CO2��ˮ�����˻�ѧ��Ӧ��
(2)ȡ3mLƿA�е�Һ�嵹���Թ�B�У��μ���ɫʯ����Һ�����۲쵽��Һ��ɫ��Ϊ____ɫ��д������ƿA�з�Ӧ�Ļ�ѧ����ʽΪ____
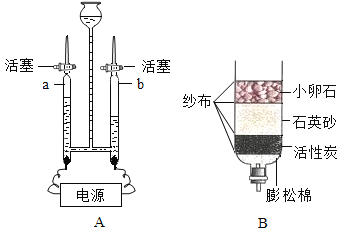
(3)��(2)���Թ�B��������ͼ����ʾ��ʵ�顣ʵ���пɹ۲쵽�Թ�B��������ð������Һ��Ϊ____ɫ���÷�Ӧ����ʽΪ____���Թ�C��������____���Թ�C�з�����Ӧ�Ļ�ѧ����ʽΪ___��
���𰸡����� �� CO2+H2O=H2CO3 �� H2CO3![]() CO2+H2O ����� Ca(OH)2+CO2=CaCO3��+H2O
CO2+H2O ����� Ca(OH)2+CO2=CaCO3��+H2O
��������
�⣺(1)���ڶ�����̼������ˮ������һ���ռ���CO2���������������ƿA�м���Լ1/3�����ˮ����������ƿ�ǣ�������ƿ�����֤��������̼��ˮ��Ӧ��
(2)������̼����ˮ��Ӧ����̼������ԣ���Ӧ�Ļ�ѧ����ʽΪ��CO2+H2O=H2CO3��������Һ��ʹʯ����Һ��ɺ�ɫ��
(3)̼��ȶ��������ֽ�����ˮ�Ͷ�����̼����ɫʯ����Һ���ɺ�ɫ��Ϊ��ɫ��������̼��ʹ�����ʯ��ˮ����ǣ��÷�Ӧ����ʽΪ��Ca(OH)2+CO2=CaCO3��+H2O��

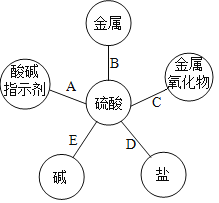
����Ŀ������е�Ԫ�ؿ�������������ʡ�
Ԫ������ | �� | ̼ | �� | �� | ͭ |
Ԫ�ط��� | H | C | O | S | Cu |
��1��ͭԪ��ͨ����![]() ��
��![]() ��,����Ԫ�ؿ���ɵ�������_____���û�ѧʽ��ʾ����
��,����Ԫ�ؿ���ɵ�������_____���û�ѧʽ��ʾ����
��2���ס��ҡ����dz��г�������,Ҳ�����ϱ��е�һ�ֻ�����Ԫ����ɵġ���֪�ס��������Ԫ����ͬ,����һ�������Һ�塣�ס��ҡ���������ͼ��ʾ�Ĺ�ϵ,��![]() ����ʾת����ϵ��
����ʾת����ϵ��
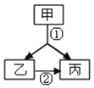
�ٱ���_____�������ƣ���
���ҵ���;��_____��дһ�֣���
��д����ת�����Һͱ������ֱ���ʽ_____��
����Ŀ������ʵ�鷽�����ܴﵽ��ӦĿ���ǣ� ��
A | B | C | D | |
ʵ�鷽�� |
|
|
|
|
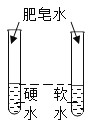
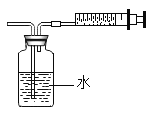
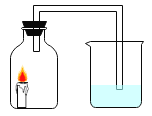
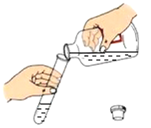
Ŀ�� | ֤�����������е� | �õ�������ˮ����Ӳˮ����ˮ | ���װ�õ������� | �ⶨ�����������ĺ��� |
A.AB.BC.CD.D