��Ŀ����
����Ŀ��ʵ���ҳ��õĸ��������ʯ�����������ƺ����������ƵĻ�����������е�ˮ�����Ͷ�����̼��Ӧ�����ʡ�ijͬѧ��һƿ���õļ�ʯ�ҽ���������̽����
����������裩
����һ��û�б��ʣ�ֻ���������ƺ��������ƣ�
����������ֱ��ʣ�
����������ȫ���ʣ�����______________��______________��
���������Ʊ��ʵĻ�ѧ��Ӧ����ʽ��______________________________��
������ʵ�飩
ʵ����ͼ��ʾ��
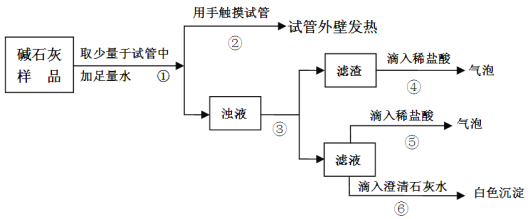
����������ۣ�
�������е������е�������________���ɲ����������жϣ�������______________�����������������������������ɲ����ݢ������ж���Һ�к���_____________���ɴ��жϲ���һ���������ó����ۡ�
����˼�����ۣ�
ʵ�����м�ʯ��Ӧ��______________���档
���𰸡��������� ̼���� CO2��2NaOH=Na2CO3��H2O ������̼��CO2�� ������ ̼���ƣ�NaCO3�� �ܷ⡢����
��������
�ټ�ʯ����Ʒȡ������ˮ�ܽ⣬�����ִ����Թ���ڣ����ȸУ�����Ʒ��һ���������ƣ�����������ˮ���ȣ���Ʒû����ȫ���ʣ��۽���Һ���˺õ���������Һ������������ϡ���������ݲ�����������һ����̼��ƣ�����һ���Ƕ�����̼���壻����Һ�м���ϡ���������ݲ���������Һ��һ����̼���ƣ�����һ���Ƕ�����̼���壻����Һ�м�������ʯ��ˮ�а�ɫ����������Һ��һ����̼���ƣ�
����������ʯ����ȫ���ʣ���������ˮ��Ϊ�������ƣ�����������������̼��Ϊ̼���ƣ� ���������Ʊ��ʵĻ�ѧ��Ӧ����ʽ��CO2��2NaOH=Na2CO3��H2O��
��������ۣ�
����������Һ�м���ϡ���������ݲ���������Һ��һ����̼���ƣ�����һ���Ƕ�����̼���壻�ɲ����������жϣ�����������������Ϊ���������ִ����Թ���ڣ����ȸУ�����Ʒ��һ���������ƣ�����������ˮ���ȣ���Ʒû����ȫ���ʣ��ɲ�������Һ�м���ϡ���������ݲ���������Һ��һ����̼���ƣ�����һ���Ƕ�����̼���壻��������Һ�м�������ʯ��ˮ�а�ɫ����������Һ��һ����̼���ƣ�˵����Ʒ�����������Ѿ����ʣ��ɴ��жϲ���һ���������ó����ۡ�
��˼�����ۣ�
ʵ�����м�ʯ����������е�ˮ�����Ͷ�����̼��Ӧ�����ʣ�Ӧ�ã��ܷ⡢���ﱣ�档

 �ִʾ��ƪϵ�д�
�ִʾ��ƪϵ�д�����Ŀ������ƾ�����ʹ�á������Я�����㣬ȼ��ʱ�Ի�������Ⱦ���٣���Һ��ƾ��Ƚϰ�ȫ�Խϸߣ���Ϊһ�ֹ���ȼ�ϣ��㷺Ӧ���ڲ���ҵ������ҵ��Ұ����ҵ�ĵȳ��ϡ���һ���ò��У�ͬѧ�ǶԹ���ƾ������˺��棬���Ƕ���ɷֽ����о���

���������ϣ���1���ù���ƾ����þƾ����Ȼ��ƺ��������ư�һ���������Ȼ���Ƴɡ���2���Ȼ��ơ��Ȼ�����Һ�������ԡ���3��Ũ������к�ǿ����ˮ�ԣ���ʯ�ҳ���������ˮ�����Ͷ�����̼��
̽���һ��̽������ƾ��ijɷ�
��������⣩��1���ƾ����Ƿ���̼Ԫ�أ�
��2������ƾ��е����������Ƿ���ʣ�
��ʵ��̽������1������ͼʵ�飬���ֳ���ʯ��ˮ����ǣ��ɴ˿ɵó��ƾ��к���̼Ԫ�صĽ��ۣ��˽���__________��ѡ�������������������

��2��ȡ��������ƾ����ձ��У���������ˮ����ܽ���ã������ձ��ײ��а�ɫ������ȡ�������Թ��м�ϡ���ᣬ�����ݲ�����д���� ������Ļ�ѧ����ʽ��______________ ������ʵ�鲢������ϵó����������ѱ��ʡ�
��3��Ϊ��һ��ȷ���������Ƶı��ʳ̶ȣ�����̽����
�ټ����ձ��ϲ���ҹ����֧�Թ��У�����ͼ̽����
���� | | |
���� | ��Һ��� | ���� |
���� | ��Һ������������ | ��ҹ����̼���� |
��������Ϊ����ʵ�鲻��֤����ҹ��һ�����������ƣ���������_______________��������ȡ�ձ����ϲ���ҹ���������Ȼ�����Һ����ַ�Ӧ��μӷ�̪��Һ����̪��Һ��졣
��ʵ����ۣ�������һ����Ϊ����ƾ��е��������Ʋ��ֱ��ʡ�
����չӦ�ã�Ҫ��ȡ���ֱ��ʵ�����������Һ�е����ʣ���ѡ��������__________��ѡ����ĸ��ţ���
ABa��OH��2��Һ BCaCI2��Һ CCa��OH��2
̽�������̽������ƾ���̼���Ƶ���������������ͼ��ʾʵ��װ�ã�����̨��ȥ�����Լ���ͨ���ⶨ��Ʒ��ϡ���ᷴӦ������CO2���������������Na2CO3������������װ�����������ã���������Ļӷ�����ÿ����Ӧ�����ö�����ȫ�ģ���
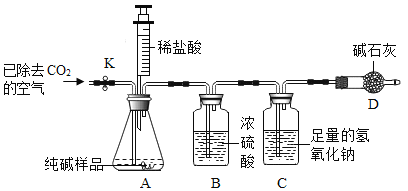
��1����ֹˮ��K���ȶ�װ��A��B�������ӣ�ͨ���ѳ�ȥCO2�Ŀ���һ��������ž�װ�õ�A��B�к��е�__________���ٽ���װ��C��D��
��2���ر�ֹˮ��K������������ϡ���ᣨ���ʲ������ᷴӦ����
��3����װ��A�еķ�Ӧ��������һ�δ�ֹˮ��K��������װ��ͨ���ѳ�ȥCO2�Ŀ���һ��������������غ㶨�ɣ�װ��_________������ţ��ڷ�Ӧǰ�����������Dz���CO2���������ɴ˼�����ù���ƾ���Na2CO3��������������û��װ��D������ʹ�ⶨ���______________��ѡ�ƫ��ƫС������
����Ŀ����ѧ��ȤС���ͬѧ��������������������������Ӽ���һ���������ᾧ�壬��һ���DZ�עΪ���εİ�ɫ��ĩ��ͬѧ�ǽ���������������ְ�ɫ��δ����ˮ����Ϻ������ʹ����ʯ��ˮ����ǵ����壬�������Ƕ�ɫ��ĩ�Ļ�ѧ�ɷֽ���������̽����
��������룩����1.̼���ƣ�����2.̼�����ƣ�����3.___________��
���������ϣ���̼������Һ��̼��������Һ���ʼ��ԣ��� ̼���������ȷֽ�����̼���ơ�ˮ�Ͷ�����̼��̼�������Ȳ��ֽ�
��ʵ��̽������ȤС����Ʋ�ͬʵ�鷽������̽����
���鷽��������ɫ��ĩ����ˮ����pH��ֽ�ⶨ�����ȣ�pH>7����1��ȷ
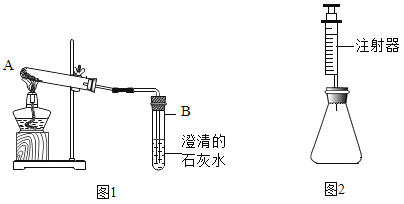
���鷽������ͼ1��ȡ������ɫ��ĩ���Թ��У��������������ʯ��ˮ�����ȣ�����ʯ��ˮ����ǣ�����2��ȷ��д��װ��B�з�Ӧ�Ļ�ѧ����ʽ______________��
���鷽��������ͼ2��ʾװ����Ϊ��Ӧ������ȷ�������������������жϰ�ɫ��ĩ�ijɷ֡��ֱ���ʢ��̼���ơ�̼�����ƺͰ�ɫ��ĩ����ƿ�У�ע����������Ũ�ȵ�������ϡ���ᣬ��¼�����
ʵ���� | ��ƿ������[��Դ | ���յõ� CO2 ���/mL | |
���� | ����/g | ||
�� | ̼���� | a | V1 |
�� | ̼������ | a | V2 |
�� | ��ɫ��ĩ | m | V3 |
ʵ��ٵĻ�ѧ��Ӧ����ʽΪ____________�� ���� m=_______g�� �����ϱ������ݷ���������3��ȷ���жϵ�������__________��
��������˼��������ۺ���Ϊ�ס�����Ľ��۶���ȷ����ȷ��ԭ����__________������_____________��
�����۷�����̽�������Ǻ˲鵽���Ӽ��ijɷ����������̼�����ƣ���������Ϊ��ɫ��ĩ�е�̼��������̼�����Ʒֽ�����ġ�
����չӦ�ã��������ʳ����·���һ��ʱ��Ҳ��ֽ����________������ţ���
A ̼�� B ��ˮ C ̼����� D ����