��Ŀ����
����Ŀ��ij��ѧ��ȤС��ͬѧ�����˼������ʯ��ˮ������������Һ��ʵ��̽��������һͬ����̽����

��������⣩��μ�����������ɫ��Һ��
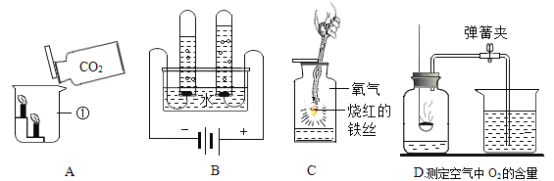
��ʵ�鷽���������ȼ�λͬѧ��������ͼ��ʾ��ʵ�飬����ش��������⣺
��1�����в��ܴﵽʵ��Ŀ�ĵ���________������ĸ��
��2��д��C��ʵ���з�����Ӧ��ʵ������_________________��
��3��д��D��ʵ��������������Ļ�ѧ����ʽ______________��
������̽����ʵ�����������ͬѧ��A��B��C��D�����Թ��е�����ȫ������ͬһ���ɾ����ձ��У���ַ�Ӧ�õ���ɫ����������Һ���Ը���Һ�ijɷ��ֽ�����̽����
��������⣩����Һ�г�ˮ����̪�������Щ���ʣ�
���������ϣ��Ȼ�����Һ�����ԣ�
����������裩��NaCl��CaCl2 ��NaCl��CaCl2��NaOH ��NaCl��CaCl2��HCl
����˼����չ��
�����������������ֻ��һ��������������________������ţ���������________��
��Ϊ����֤�ձ�����Һ�п����е������Ƿ���ڣ�ʹ��������Щ���ʿ��ԴﵽĿ����________������ĸ����
A ������ B ͭ C ��������Һ D ��������������Һ
���𰸡�AB ����һ֧�Թܲ�����ɫ��������һ֧�Թ������������� CO2+ Ca(OH)2 =CaCO3��+ H2O �� ��Һ�к��з�̪��Һ����ַ�Ӧ�õ���ɫ����������Һ��˵����Һ���Լ��� A
��������
[ʵ�鷽��]��1�����������ʯ��ˮ������������Һ��Ӧ����������������𣻳���ʯ��ˮ������������Һ���Լ��ԣ�����ʹ��̪��Һ��죬�����÷�̪��Һ����̼������Һ�����ʯ��ˮ��Ӧ���ɰ�ɫ������������������Һ����Ӧ����������̼������Һ���𣻶�����̼��ʹ����ʯ��ˮ����ǣ�������������Һ��Ӧ�������ö�����̼���𡣹����в��ܴﵽʵ��Ŀ�ĵ���AB����2��C��ʵ���е�̼������Һ�����ʯ��ˮ��Ӧ������ɫ��������3��D��ʵ���еĶ�����̼�����ʯ��ˮ��Ӧ����̼��ư�ɫ������ˮ����Ӧ�Ļ�ѧ����ʽΪ��CO2+ Ca(OH)2 =CaCO3��+ H2O
[��˼����չ]
�ٽ�A��B��C��D�����Թ��е�����ȫ������ͬһ���ɾ����ձ��У���Һ�к��з�̪��Һ����ַ�Ӧ�õ���ɫ����������Һ��˵����Һ���Ǽ��Եģ��ʲ����������Ϊ����֤�ձ�����Һ�п����е������Ƿ���ڣ�ʵ������֤�����Ƿ���ڡ�ʹ��������Щ���ʿ��ԴﵽĿ����________������ĸ����
A ��������������Ӧ�����Ȼ�����ɫ��Һ�����������ڣ���Һ����ɫ��Ϊ��ɫ����ѡ��������⣻B ͭ�������Ӧ������֤�����Ƿ���ڣ���ѡ��������⣻C ��������Һ���Ȼ�����Һ���Ȼ�����Һ�����ᶼ�ܷ�Ӧ������ɫ������������֤�����Ƿ���ڣ���ѡ��������⣻D ��̪��Һ�����ԡ�������Һ����ɫ���ù�������������Һ��������Һ�Լ��ԣ���̪��Һ����죬������֤�����Ƿ���ڣ���ѡ��������⡣��ѡA��

 ��У����ϵ�д�
��У����ϵ�д�����Ŀ������������������ͬ�������Ϲ�ϵ���С�
��һ�������Ĺ㷺Ӧ��
��1����������ͨ����Ϊ�����֣����������Ͻ����Ҫ���������______________��
��2������Ӧ���������ý��������Ե���_____________����д��ĸ��ţ���

��������������ʴ������
��1���������������Ʒ�����������________________������ţ���
a��ʪ������ b��������� c���ֽ���ʳ��ˮ��
��2��д����ϡ���������Ļ�ѧ����ʽ______________��
������������ɷ����ⶨ
��1��������һ����![]() �����ܺ�
�����ܺ�![]() ����ij������Ʒ�м�ϡ���ᣬ_____________��������֤������
����ij������Ʒ�м�ϡ���ᣬ_____________��������֤������![]() ��
��
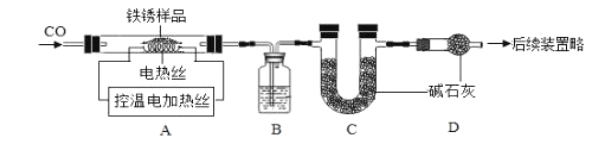
��2����ȡ23.2gֻ��![]() ������������Ʒ������ͼװ��ʵ�顣
������������Ʒ������ͼװ��ʵ�顣
���������ϣ�a.��110��ʱ��![]() ��ȫ�ֽ�Ϊ
��ȫ�ֽ�Ϊ![]() ��
��![]() ��
��
b.500��ʱ![]() �ſ�ʼ����ԭ�����¶Ȳ�ͬʱ���������
�ſ�ʼ����ԭ�����¶Ȳ�ͬʱ���������![]() ����ɫ����
����ɫ����![]() ��
��
����װ���м���������Ʒǰ������еIJ�����________________��
��ʵ��ʱ����ͨ��CO��Ŀ����________________��
�۵����ȳ���500��ʱ���۲쵽A�е�������________________��
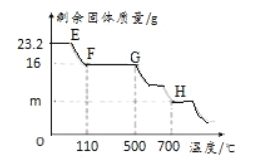
���±�Ϊ������700��ʱ�������ݣ���ͼΪA�й��������ͼ����¶ȹ�ϵͼ
��Ӧǰ | ��Ӧ�� | |
װ��B/g | 100.0 | 107.2 |
װ��C/g | 80.0 | 84.4 |
�ش���������
a. ![]() ��n��ֵΪ________________������װ��D��������n��ֵ________________������
��n��ֵΪ________________������װ��D��������n��ֵ________________������
b. H���Ӧ�Ĺ���Ϊ������仯ѧʽΪ________________��д��������̣���