��Ŀ����
����Ŀ����ѧ������ϢϢ��أ�
(1)�±���100gijʳƷ�IJ���Ӫ���ɷ֣�
������ | ������ | ���� | ��֬ | ˮ | �� | �� | �� |
2060kJ | 50g | 20g | 20g | 5g | 5.6mg | 3.3mg | 8mg |
��ʳƷ��û���г���Ӫ������______
(2)Ϊ�˷�ֹ��ͯ���Ͳ����������븻��____Ԫ�ص�ʳ�
(3)����������ɱ��ϸ���������ͼ������ѵȣ������׳�Ϊ_______��
(4)����������һЩʳ��Ľ���pH(����)�����гʼ��Ե���____(�����)��
pH | 3.5��4.5 | 2.9��3.3 | 6.3��6.6 | 7.6��8.0 |
ʳ�� | A������֭ | B��ƻ��֭ | C��ţ�� | D�������� |
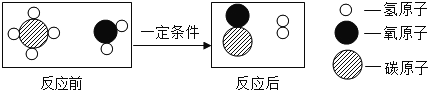
(5)Ϊ�����������������̼���ŷţ����Խ�������̼����ת������ͼΪ�÷�Ӧ����ʾ��ͼ������ͼʾ�ش��������⣮
��Ӧǰ | ��Ӧ�� |
| ||
A | B | C | D | |
|
|
|
| |
������C����______����(��������������ԭ��������������)��
������4���������������������______(����ĸ���)��
�۸÷�Ӧ��C��D�������ʵ���������_____��Dȼ�յĻ�ѧʽ��______
���𰸡�ά���� �� �ռ�(����������) D ���� AC 9��4 CH4+2O2![]() CO2+2H2O
CO2+2H2O
��������
��1������Ӫ���أ�ˮ�����Ρ����̼ࣨˮ���������֬�������ʡ�ά���أ���ʳƷ��û���г���Ӫ�����ǣ�ά���أ�
��2����ͯ��Ҫ��Ԫ�أ�Ӱ������������������������Ͳ���������ȱ��Ԫ�����������ɣ�
��3�������������������ռ�����ƣ�
��4��pH��7���ʼ��ԣ�������pH��Χ7.6~8.0���ڼ��ԣ���ѡD��
��5�����۷�Ӧʾ��ͼ��֪��A+B��C+D����������CO2��4H2 2H2O��CH4,
2H2O��CH4,
������C��ˮ��ˮ��ˮ���ӹ��ɣ�
�ڶ�����̼��ˮ���������
�۸÷�Ӧ��ˮ�ͼ���������ȣ�2��18������12+4��=9:4������ȼ�յķ�Ӧԭ����CH4+2O2![]() CO2+2H2O��
CO2+2H2O��

 Ӯ�ڿ�����ʦ��ʱ�ƻ�ϵ�д�
Ӯ�ڿ�����ʦ��ʱ�ƻ�ϵ�д� �������Ͽ�ʱͬ��ѵ��ϵ�д�
�������Ͽ�ʱͬ��ѵ��ϵ�д�