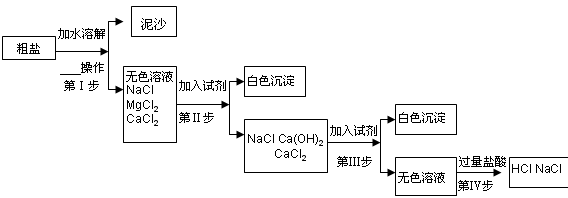
��Ŀ����
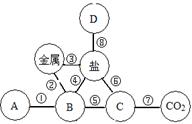
���й����Ͻ��ܣ������ǣ���Ҫ�ɷ�̼��ƣ��������ľ�ң���Ҫ�ɷ�̼��أ���ˮ�����ã����Եõ��������ء�ij��ѧ��ȤС�飬Ϊ����ȡ�������أ�������̼��Ƴ�����Ⱥ�Ĺ�������ձ��У��������м���һ������10%��̼�����Һ����ַ�Ӧ��ȴ����ˡ�ϴ�ӵõ�����A����ҺB����ش������й����⣺
��1��ȡ��������A���Թ��У�����ϡ���ᣬ�����ݲ�����֤��A�к���__________��
��2��ȡ��������A���Թ��У���ˮ����ܽ⡢���ˣ�����Һ�еμ�______________����Һ���ɫ��֤��A�л������������ơ�
��3����ϣ�1����2������A�ɷֵ�ȷ���������ƶ���ҺB�ijɷ����������غ��������ơ�Ϊ�˵õ��������������أ�������ҺB�еμ�������_____________��Һ��������Ӧ�Ļ�ѧ����ʽΪ____________________________________________����ַ�Ӧ����ˣ�����Һ���ɿ��Ƶýϴ������������ء�
��1��ȡ��������A���Թ��У�����ϡ���ᣬ�����ݲ�����֤��A�к���__________��
��2��ȡ��������A���Թ��У���ˮ����ܽ⡢���ˣ�����Һ�еμ�______________����Һ���ɫ��֤��A�л������������ơ�
��3����ϣ�1����2������A�ɷֵ�ȷ���������ƶ���ҺB�ijɷ����������غ��������ơ�Ϊ�˵õ��������������أ�������ҺB�еμ�������_____________��Һ��������Ӧ�Ļ�ѧ����ʽΪ____________________________________________����ַ�Ӧ����ˣ�����Һ���ɿ��Ƶýϴ������������ء�
��1��CaCO3 ��2����̪��Һ
��3��K2 CO3��2�֣��� Ca(OH)2 + Na2 CO3 = CaCO3��+ 2 Na OH
��3��K2 CO3��2�֣��� Ca(OH)2 + Na2 CO3 = CaCO3��+ 2 Na OH
���㣺
�������������е�֪ʶ���з�����̼���������ᷴӦ�������壬��̪��Һ�ڼ�����Һ�б�죬������������̼��ط�Ӧ����Ӧ���еij̶������ʵ����йأ����ܴ������ʹ���������
��𣺽⣺��1��ȡ��������A���Թ��У�����ϡ���ᣬ�����ݲ�����֤������A�к���̼��ƣ����CaCO3��
��2��ȡ��������A���Թ��У���ˮ����ܽ⡢���ˣ�A�л������������ƣ�˵����Һ�ʼ��ԣ���ʹ��̪��Һ��죬�ʵμӵ��Լ��Ƿ�̪��Һ�������̪��Һ��
��3��Ҫ�õ��������������أ���Ҫ����̼�����Һ���������Ʒ�Ӧ��̼��������������Ʒ�Ӧ����̼��Ƴ������������أ����K2CO3��Ca��OH��2+K2CO3=CaCO3��+2KOH��
���������⿼���˻����ɷֵ��ƶϣ���ɴ��⣬������������ṩ����Ϣ������ʵ����ʽ��У�
�������������е�֪ʶ���з�����̼���������ᷴӦ�������壬��̪��Һ�ڼ�����Һ�б�죬������������̼��ط�Ӧ����Ӧ���еij̶������ʵ����йأ����ܴ������ʹ���������
��𣺽⣺��1��ȡ��������A���Թ��У�����ϡ���ᣬ�����ݲ�����֤������A�к���̼��ƣ����CaCO3��
��2��ȡ��������A���Թ��У���ˮ����ܽ⡢���ˣ�A�л������������ƣ�˵����Һ�ʼ��ԣ���ʹ��̪��Һ��죬�ʵμӵ��Լ��Ƿ�̪��Һ�������̪��Һ��
��3��Ҫ�õ��������������أ���Ҫ����̼�����Һ���������Ʒ�Ӧ��̼��������������Ʒ�Ӧ����̼��Ƴ������������أ����K2CO3��Ca��OH��2+K2CO3=CaCO3��+2KOH��
���������⿼���˻����ɷֵ��ƶϣ���ɴ��⣬������������ṩ����Ϣ������ʵ����ʽ��У�

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ
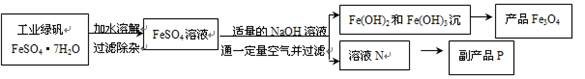
 Fe3O4 + 4H2O��
Fe3O4 + 4H2O��