��Ŀ����
����Ŀ����ͼ��ʾΪʵ�����г����������Ʊ�������������ռ�������ʵ��IJ���������

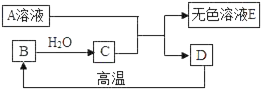
����װʵ��װ��ʱ�����ظ�ѡ����������ij��ѧС���ͬѧ��������������и�̽��ʵ�顣
��1��Сӱͬѧ�Թ���������Һ�Ͷ�������Ϊԭ�ϣ�����ʵ�������Ʊ����ռ������������ ������Ҫ�����ʵ��װ�á�������������������װ�õ������ԡ�
������ѡ����������˳��ӦΪ___________������������д���������ĸ����
���ù���������Һ�Ͷ���������ȡ�����Ļ�ѧ����ʽΪ___________��
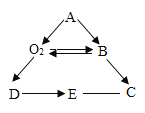
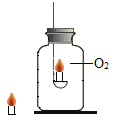
��2��С÷ͬѧѡ��������װ�� A��G��H ����ij�������ʵ�̽��ʵ�顣ʵ������У����۲쵽װ�� G �е���Һ���ɫ���ǣ�װ�� H �еĵʹ�������Ϩ�𣬸ߴ������Ϩ��
��С÷ͬѧ��װ�� A �м����ҩƷ���ӦΪ___________������ţ���
A ��ʯ�Һ�ϡ���� B ��ʯ�Һ�ϡ���� C ʯ��ʯ��ϡ���� D п����ϡ����
��װ�� A �з�����Ӧ�Ļ�ѧ����ʽΪ___________��
�۸�������ʵ����Ϣ�������йظ��������ʵ�˵���У��������___________������ţ���
A ��������������������Һ��Ӧ���ɰ�ɫ���� B ͨ��״���£��������ܶȴ��ڿ���
C �����岻��ȼ���Ҳ�֧��ȼ�� D ͨ��״���£��������ܶ�С�ڿ���
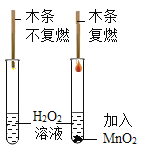
��3��С·ͬѧ���ij��������Ʒ�����Ԫ�ؽ���̽��ʵ�飨������ʾ������һ���� C��H Ԫ�أ����ܺ���Ԫ�أ�����ѡ���˲�������װ�ã�������A��C1��B��C2��D����ʯ�Ҹ��������˳�����ӡ�
����֪��װ�� A ���Լ�Ϊ H2O2 ��Һ�� MnO2 ��װ�� C1��C2 Ϊʢ��Ũ�����ϴ��ƿ��������ʵ��ǰ������װ�������ԣ�Ȼ��ʹһ�����ĸ�������Ʒ��м�ڴ������г��ȼ�գ��۲�����ȷ�ռ��˸�ʵ����й����ݡ����������з����Ļ�ѧ��Ӧ����ֽ��У�
����֪װ�� B �Ĵ������ڷ����� 6.0g ����������м�������������ȼ�պ��װ�� C2 �� ���������� 3.6g��װ�� D ������������ 8.8g���������������Ԫ�����Ϊ___________��
��װ�� C1 ��������___________��
��������ʵ���в�����װ�� C1���⽫���������Ʒ���Ԫ�ص�ʵ��ⶨ�������������Ӱ��___________��
���𰸡�A��C��F 2H2O2![]() 2H2O + O2�� C CaCO3 + 2HCl = CaCl2 + H2O + CO2�� D C��H��O���� ̼���⡢��Ԫ�أ� ������������ ��ȥ O2 �е�ˮ��������ֹӰ��װ�� C2 ���زⶨ �Ⱥ����𰸣� ��������װ�� C1 ���ᵼ��װ�� C2 ��������ƫ���ɴ˼��������Ԫ������ƫ�� ����Ӱ����Ԫ�ص�������������Ԫ�������жϣ�����Ʒ̼Ԫ�������IJⶨ��Ӱ�졣
2H2O + O2�� C CaCO3 + 2HCl = CaCl2 + H2O + CO2�� D C��H��O���� ̼���⡢��Ԫ�أ� ������������ ��ȥ O2 �е�ˮ��������ֹӰ��װ�� C2 ���زⶨ �Ⱥ����𰸣� ��������װ�� C1 ���ᵼ��װ�� C2 ��������ƫ���ɴ˼��������Ԫ������ƫ�� ����Ӱ����Ԫ�ص�������������Ԫ�������жϣ�����Ʒ̼Ԫ�������IJⶨ��Ӱ�졣
��������
��1�����Թ���������Һ�Ͷ�������Ϊԭ�ϣ�����ʵ�������Ʊ����ռ������������Ӧѡ�õķ���װ��Ϊ��Һ��ϳ����ͷ���װ�ã�Ӧ��Ũ������и��Ȼ���������ſ������ռ���������ѡ����������˳��ӦΪ��A��C��F��
�ڹ���������Һ�ڶ������̵Ĵ��·ֽ⣬����ˮ����������Ӧ�Ļ�ѧ����ʽΪ��2H2O2![]() 2H2O + O2����
2H2O + O2����
��2��װ�� G �е���Һ���ɫ���ǣ�װ�� H �еĵʹ�������Ϩ�𣬸ߴ������Ϩ��˵�����ɵ������Ƕ�����̼��
��ʵ������ȡ������̼��ҩƷ���Ϊ������ʯ����ʯ��ʯ����ϡ���ᣬС��÷ͬѧ��װ�� A �м����ҩƷ���ӦΪ��C��
��ʯ��ʯ����Ҫ�ɷ���̼��ƣ�̼�����ϡ���ᷴӦ�����Ȼ��ơ�ˮ�Ͷ�����̼����Ӧ�Ļ�ѧ����ʽΪ��CaCO3 + 2HCl = CaCl2 + H2O + CO2����
����װ��G�е������֪����������������������Һ��Ӧ���ɰ�ɫ��������װ��H�е������֪�������岻��ȼ���Ҳ�֧��ȼ�գ��ܶȴ��ڿ�������ѡD��
��3����װ�� B �Ĵ������ڷ����� 6.0g ����������м�������������ȼ�պ��װ�� C2 ������������ 3.6g��˵������ˮ������Ϊ3.6g��װ�� D ������������ 8.8g��˵�����ɶ�����̼������Ϊ8.8g��3.6gˮ����Ԫ�ص�����=3.6g��![]() =0.4g��8.8g������̼��̼Ԫ�ص�����=8.8g��
=0.4g��8.8g������̼��̼Ԫ�ص�����=8.8g��![]() =2.4g��0.4g+2.4g��6.0g���ʸ�����������Ԫ�����ΪC��H��O���� ̼���⡢��Ԫ�أ���
=2.4g��0.4g+2.4g��6.0g���ʸ�����������Ԫ�����ΪC��H��O���� ̼���⡢��Ԫ�أ���
��װ�� C1 �������Ǹ����������� ��ȥ O2 �е�ˮ��������ֹӰ��װ�� C2 ���زⶨ�ȣ���
����������װ�� C1 ���ᵼ��װ�� C2 ��������ƫ���ɴ˼��������Ԫ������ƫ����Ӱ����Ԫ�ص�������������Ԫ�������жϣ�����Ʒ̼Ԫ�������IJⶨ��Ӱ�졣

 Ӣ��СӢ������Ĭдϵ�д�
Ӣ��СӢ������Ĭдϵ�д� �����ҵ���������ͯ������ϵ�д�
�����ҵ���������ͯ������ϵ�д�����Ŀ���±��� NaHCO3��NH4Cl�ڲ�ͬ�¶��µ��ܽ�ȡ�����˵������ȷ���ǣ�������
�¶�/�� | 20 | 30 | 40 | 50 | |
�ܽ��/g | NaHCO3 | 10.0 | 11.0 | 13.0 | 14.0 |
NH4Cl | 35.0 | 39.0 | 43.0 | 48.0 | |
A.20��ʱ�����Ƶ������� NaHCO3��NH4Cl������Һ��NH4Cl��Ҫˮ��
B.���Ͱ���ˮ����CO2�����ɵ� NaHCO3��NH4Cl��NaHCO3�Ƚᾧ����
C.20��ʱ��NaHCO3������Һ��������������Ϊ10%
D.50��ʱ��148g NH4Cl������Һ���µ�20�棬����13 g NH4Cl����
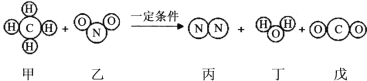
����Ŀ����һ�ܱ���������������������ˮ������һ�ֳ��л�ѧѧϰ��������W����һ�������³�ַ�Ӧ����÷�Ӧǰ������ʵ����������ʾ������˵���в���ȷ���ǣ�������
���� | W | ���� | ���� | ˮ���� |
��Ӧǰ����/g | 68 | 100 | 2 | 2 |
��Ӧ������/g | X | 4 | 58 | 110 |
A.X��ֵΪ0
B.��Ӧǰ��Ԫ�صĻ��ϼ۷����˸ı�
C.W�к�����Ԫ��
D.���ɵĵ�����ˮ��������֮��Ϊ14:27