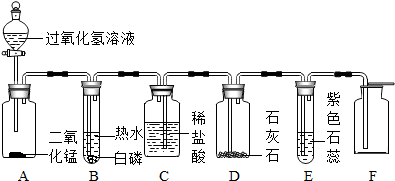
��Ŀ����
ij��ѧʵ��С��ͬѧ��Ϊ�˼�Ȿ��ij�����¹��������������ɷ�ʱ�����ǽ�������̽�����������벢����й����⣺��һ���õ�����ƽ��ȡ����ӹ�ʱ���������м��Ʒ10g�����ձ��У�
���������ձ��м���90gϡ������þƾ��ƽ��м��ȣ�һ��ʱ���ֹͣ���ȣ�
���������ձ��ڵ�������ȴ���ֽ����˳������������ձ��е����˼���ʯ����Һ��
��������ʵ�鷢�֣�
�ٷ�Ӧ���ձ������ʵ�������ʣ��99.66g��
���ձ��ڵ�Һ����ʹʯ����Һ��ɺ�ɫ��
���ձ��ײ���ʣ���������ڵĹ������ʣ�
����ʵ�鷢�ֿ�֪��
��1���ձ��ײ����������ڹ������ʵ���Ҫ�ɷ���
��2�����������ʲ��μӷ�Ӧ�������������к����������������Ƕ��٣�
��3������������100t����������������Ҫ����������������Ϊ80%�ij�������ٶ֣�
��������1������������һ�ֺϽ𣬺�̼��Ϊ2%-4.3%��̼���Ǻ�ɫ���壻
��2������ȷ����⣬����ݷ�Ӧ�Ļ�ѧ����ʽ���ó�������֮��������ȣ��г�����ʽ�����ɼ��������������������������ķ�����
��3�����ݺ�����һ�����г�����ʽ���㼴�ɣ�
��2������ȷ����⣬����ݷ�Ӧ�Ļ�ѧ����ʽ���ó�������֮��������ȣ��г�����ʽ�����ɼ��������������������������ķ�����
��3�����ݺ�����һ�����г�����ʽ���㼴�ɣ�
����⣺��1����Ϊ�����к���̼���ʷ�Ӧ���ձ��ײ�����ʣ���������ڵĹ���������̼�ۣ���̼�����ʴ�Ϊ��̼�ۣ���̼����
��2���⣺��������Ʒ�к���������������Ϊx%
Fe+H2SO4=FeSO4+H2����1�֣�
56 2
10g��x% 100g-99.66g
=
��x=95.2%��
�𣺸����������к�����������������95.2%��
��3���⣺������100t��������������������Ϊy
100��95.2%=y��(80%��
��100%����y=170t��
��������Ҫ����������������Ϊ80%�ij�����170�֣�
��2���⣺��������Ʒ�к���������������Ϊx%
Fe+H2SO4=FeSO4+H2����1�֣�
56 2
10g��x% 100g-99.66g
| 56 |
| 10g��x% |
| 2 |
| 0.34g |
�𣺸����������к�����������������95.2%��
��3���⣺������100t��������������������Ϊy
100��95.2%=y��(80%��
| 112 |
| 160 |
��������Ҫ����������������Ϊ80%�ij�����170�֣�
������������Ҫ����ѧ�������������ʵ��˽⣬�Լ����û�ѧ����ʽ���м����������

��ϰ��ϵ�д�
�����Ŀ
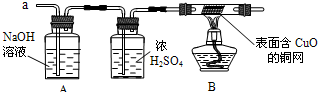
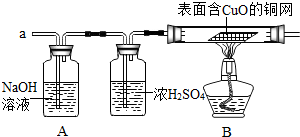 ��1�������������̽����ij��ѧʵ��С��ͬѧ������Ͷ������ͭ��Һ�У��������ɺ�ɫ�������ʵ�ͬʱ�н϶�����ݷų�����һ������ͬѧ�ǵ�̽�����������ɵ���ʲô���壿
��1�������������̽����ij��ѧʵ��С��ͬѧ������Ͷ������ͭ��Һ�У��������ɺ�ɫ�������ʵ�ͬʱ�н϶�����ݷų�����һ������ͬѧ�ǵ�̽�����������ɵ���ʲô���壿