��Ŀ����
����Ŀ�����������ҹ�������ҵ���ٷ�չ��
��1��2016��11��3�գ�����5�Ŵ��������ػ���ɹ����䡣�û��ʹ��Һ��Һ�����ƽ�����ȼ��ȼ�յĻ�ѧ����ʽΪ___________��
��2��2016��11��18�գ�����ʮһ�����˷ɴ�˳�����ء��ɴ����ز����ط�������ʱ�������������Ħ������������¶Ȼἱ����������ˣ����زձ�����������õķ����´�ʩ�������йط��ز��⸲�Dz��ϵ�˵����������__________ (�����)��

a���������£�����������е�������������ȼ��
b���������£��ܷ������ȷ�Ӧ��������
c���������£����ۻ���������������
d�������ԣ�����������������
���𰸡�2H2 +O2![]() 2H2O bcd
2H2O bcd
��������
��1��2016��11��3�գ�����5�Ŵ��������ػ���ɹ����䡣�û��ʹ��Һ��Һ�����ƽ�����ȼ��ȼ������ˮ����Ӧ�Ļ�ѧ����ʽΪ2H2 +O2![]() 2H2O��
2H2O��
��2��a ���������£����ز��⸲�Dz��ϲ�����������е�������������ȼ�գ���a����
b ���������£����ز��⸲�Dz����ܷ������ȷ�Ӧ������������b��ȷ��
c ���������£����ز��⸲�Dz��Ͽ��ۻ�����������������������ȷ��
d ���ز��⸲�Dz����������ԣ����������������ڣ���d��ȷ����ѡbcd��

����Ŀ��ij��ѧ��ȤС����ͨ��ʵ��ⶨij��ʯ��ʯ��̼��Ƶ�����������ȡ��ʯ��ʯ��Ʒ8.0g����80mLϡ������Ĵμ��룬�����������±�����֪��ʯ��ʯ�е����ʲ�����ˮ��Ҳ����ϡ���ᷴӦ����
ʵ����� | 1 | 2 | 3 | 4 |
����ϡ����������mL�� | 20 | 20 | 20 | 20 |
��ַ�Ӧ��ʣ������������g�� | 5.5 | m | 1.2 | n |
��1���ϱ��У�m��_____��n��_____��
��2����ʯ��ʯ��̼�Ƶ���������Ϊ_____��
��3����Ҫ��ȡ6.6g������̼��������Ҫ��ʯ��ʯ���ٿˣ�_____��д��������̣������ȷ��0.lg��
����Ŀ������������������������й㷺Ӧ�á�ʵ��С��Ը�����صĸ�ʴ�Խ����о���
������ʵ�飩
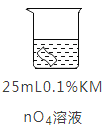
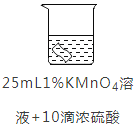
ʵ��1:�����ʼ�Ƥ�ڲ�ͬŨ�ȵ�KMnO4��Һ�н�����ͬʱ�䣬�������±���
KMnO4��ҺŨ�� | 0.002% | 0.01% | 0.1% | 1% |
��Ƥ�ı仯 | �����Ա仯 | ��Ե���ֱ�Ϊ�ػ�ɫ | ȫ����Ϊ��ɫ | ȫ����Ϊ��ɫ |
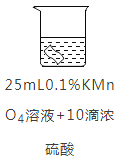
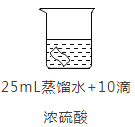
ʵ��2:��ͭƬ�ֱ������4����Һ�н���ʵ�飬�����������±���
��� | �� | �� | �� | �� | |
ʵ�� |
|
|
|
| |
ͭƬ����/g | ʵ��ǰ | 0.54 | 0.54 | 0.54 | 0.54 |
18Сʱ�� | 0. 54 | 0. 52 | 0. 54 | 0. 43 | |
����������ۣ�
��
��2��ʵ��2�У����ʵ��۵�Ŀ����__________��
��3��ʵ��2�У�ͨ���ԱȢں͢ܣ��ó��Ľ�����__________��
��4��ʵ��2�У����ó���KMnO4�����Ṳͬ���ö�ͭ���и�ʴ�����Ľ��ۣ���Ҫ�Ա�_________ (����)��
��5��ʵ��2�У�ͭƬ����ʴ�ķ�Ӧ���£���ȫ�÷�Ӧ�Ļ�ѧ����ʽ��5Cu + 2KMnO4+ 8H2SO4=5CuSO4 + 2MnSO4 + K2SO4+_________
����Ŀ��Ϊ�˲ⶨij��ͭ��ͭп�Ͻ���Ʒ����ɣ�ij��ѧ��ȤС���ͬѧ����������ʵ�飺ȡ�ķ���ͬ��������Ʒ�ֱ������������ձ��У�Ȼ��ֱ����ϡ���ᣬ��ַ�ӳ������ƽ����������������������
������� | ��1�� | ��2�� | ��3�� | ��4�� |
��ȡ��Ʒ������/g | 50.0 | 50.0 | 50.0 | 50.0 |
����ϡ���������/g | 20.0 | 40.0 | 60.0 | 80.0 |
�������������/g | 0.2 | 0.4 | 0.5 | 0.5 |
��ش��������Ⲣ���㣺
��1��50g��Ʒ��________gϡ����ǡ����ȫ��Ӧ��
��2��������Ʒ��п����������_______��д�����������̣�
��3������ͼ�л�������ϡ�������������������������ı仯��ϵ_______��