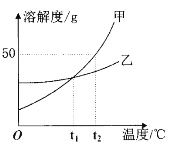
��Ŀ����
����Ŀ������кͷ�Ӧ�dz��г����ķ�Ӧ��
��1��д��������Ӧ�Ļ�ѧ����ʽ_____��
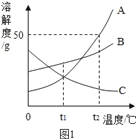
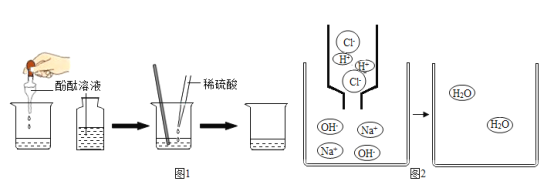
��2��������������Һ�еμ����ᣬǡ����ȫ��Ӧʱ����ʾ��ͼ��ͼ2������ͼ�л�����Ӧ����Һ�д��ڵ�����_____��
��3���������������Ҳ���Խ�������ʵ�飬ȡ��ͬŨ�ȣ���λ����ں�����ķ�������ͬ�������������ᣬ̽����NaOH��Һ����������Ӧ�������������
ʵ���� | ������� | NaOH��Һ����� | ��Ӧ�¶�/�� | ��Һ��ɫʱ�������/mL |
�� | ���� | 10mL | 25 | 2 |
�� | ���� | 10mL | 25 | 4 |
�� | ���� | 10mL | 45 | 5 |
����������ʵ���У�����ͬʱ����Ҫ���Ƶ�һ�������ǣ�_____����ͬ�����£������������Һ���٣�ԭ������ͬŨ����ͬ���ʱ��������Һ���и����_____���û�ѧ���������ȽϢں͢۷��֣������������䣬�¶�����ʱ��Ҫʹ��Һ��ɫ��Ҫ�����������ᣬԭ���������_____��
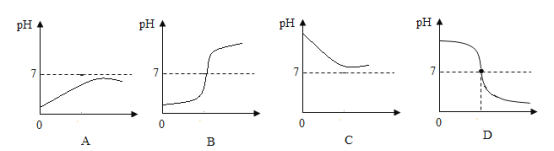
��4��С��ͬѧ�����һ���Լ������˳���������ձ���ʢ10mLϡ���ᣬ���뼸�η�̪��Һ���õι���μ�������������Һ�������Ͻ��裬������Һ��죬���������ܹ���ʾ��һ��������ҺpHֵ�仯������____��
���𰸡�NaOH��HCl=NaCl��H2O 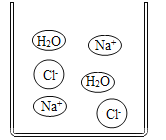 NaOH��Һ��Ũ����ͬ H+ �¶����ߣ������е������Ȼ�����ӷ�������������Ũ�ȼ��� B
NaOH��Һ��Ũ����ͬ H+ �¶����ߣ������е������Ȼ�����ӷ�������������Ũ�ȼ��� B
��������
��1������������ϡ���ᷴӦ�����Ȼ��ƺ�ˮ�Ļ�ѧ����ʽNaOH��HCl=NaCl��H2O��
��2��������������Һ�еμ����ᣬǡ����ȫ��Ӧʱ����ʾ��ͼ��ͼ2��ͼ�л�����Ӧ����Һ�д��ڵ������������ӡ������Ӻ�ˮ���ӣ���ͼ��
��3��ͬʱ����Ҫ���Ƶ�һ�������ǣ�ʹNaOH��Һ��Ũ����ͬ����ͬ�����£������������Һ���٣�ԭ������ͬŨ����ͬ���ʱ��������Һ���и���������ӣ�H�������ȽϢں͢۷��֣������������䣬�¶�����ʱ��Ҫʹ��Һ��ɫ��Ҫ�����������ᣬԭ����������¶����ߣ������е������Ȼ�����ӷ�������������Ũ�ȼ��٣���Ҫ����ϡ�����������ࣻ
��4��С��ͬѧ�����һ���Լ������˳���������ձ���ʢ10mLϡ���ᣬ���뼸�η�̪��Һ��ʱ��Һ�����ԣ�pHС��7���õι���μ�������������Һ�������Ͻ��裬������Һ��죬˵����Һ�Լ��ԣ��������ƹ�����pH����7����ҺpHֵ��С���
���������ܹ���ʾ��һ��������ҺpHֵ�仯���ǣ�B��

 ��У����ϵ�д�
��У����ϵ�д�����Ŀ���±���ij���ҹ����ֳ��п��������ܱ����Ķ����ش��������⣺
���� | ��Ⱦָ�� | ��Ҫ��Ⱦ�� | ������������ |
���� | 92 | TSP | �� |
�Ϻ� | 74 | NOx | �� |
���� | 76 | TSP | �� |
���� | 98 | SOx | �� |
ע��TSP�������е�Ʈ����NOx�����������SOx����������
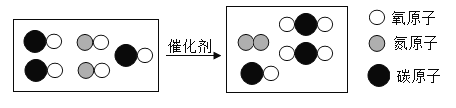
��1������������ĵ�������������е�Ԫ�غ���Ԫ�ػ��ϼ۶�Ϊ+4����д��������������Ļ�ѧʽ_____��
��2��Ϊ�˸��ƿ����������ҹ��ѿ�ʼ�ڲ��ֵ���������������������ƾ���д���ƾ�ȼ�յĻ�ѧ����ʽ��_____��
��3������β��������CO��SO2��NO�����ʣ��dz��п�������Ⱦ������ķ���֮һ������������������װ��ת���������ص���ʹCO��NO��Ӧ�����ɿɲ��������̬����ѭ���������壬��Ӧ���̿�����ͼ��ʾ���ݴ�д��������Ļ�ѧʽ_____��