��Ŀ����
����Ŀ���Ƽ��仯�����������������о��й㷺��Ӧ�á�
��һ���ƵĹ㷺��;
��1���߸����к��ƣ�����ĸ���ָ_____����ѡ����ţ���ͬ��
a������ b��Ԫ�� c��ԭ��
��2�����и�Ԫ����Ҫ�����ڹ����������У����ǻ������[Ca10(PO4)6(OH)2]��ʽ���ڣ�������Ԫ�ػ��ϼ�Ϊ___________��
��3����������Ҳ����һ�����ĺ������ʣ��ֽ������������γɸֽ�����������������в��漰�IJ�����____��
a�������� b���ϳɲ��� c�����ϲ���
����������CaCO3���Ʊ�
ijʵ��С������ʯ��ʯ���������ʲ�����ˮҲ�������ᣩ�Ʊ�����CaCO3��ͬʱ�õ�K2SO4���������£�

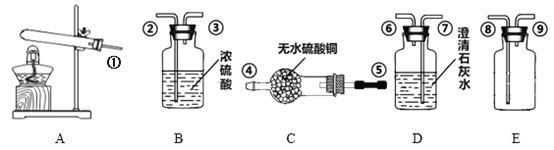
��1����Ӧ������ͨNH3����ͨCO2��Ŀ����_______����ѧ����ʽΪ______��
��2����Ӧ����������ʵ��ܽ�����±�������Ϊ��Ӧ���ڳ�������ʵ�ֵ�ԭ����______��
���� | KCl | K2SO4 | NH4Cl | M |
�ܽ��/g��25�棩 | 34.0 | 11.1 | 37.2 | 19.5 |
��3���ж�CaCO3������ϴ�Ӹɾ��ķ�����____________________��
K2SO4����ϴ��ʱ������ѡ������________ϴ�Ӽ�������ĸ��ţ���
A����ˮ B����ˮ C������K2SO4��Һ
������CaSO4xH2O�ɷֲⶨ
���������з������õ�CaSO4xH2O�к���һ������CaCO3��ʵ��С��Ϊ�˲ⶨCaSO4xH2O��x��ֵ��������ȡ22.20g��Ʒ������900�棬���õ���������ͨ��Ũ���ᣬ��ͨ���ʯ�ң����ʵ���������±���
���� | Ũ���� | ��ʯ�� |
ʵ��ǰ | 100.00 | 100.00 |
ʵ��� | 103.60 | 102.20 |
�����ϡ���֪CaSO4xH2O��160������CaSO4�� CaCO3��900��ʱ�ֽ���ȫ��
��1��CaSO4xH2O������Ϊ_____��
��2��x��ֵ___________��
���ģ�CaSO4xH2O������̽��
CaSO4xH2O���Ȼ���ʧȥ�ᾧˮ��1350��ʱ CaSO4��ʼ�ֽ⣻ȡ����CaSO4xH2O����3.44g�����ȣ��ⶨ�����������¶ȵı仯�������ͼ��ʾ��

(1)0��T1��������û�з����仯��ԭ�������_____________��
(2)G�����Ļ�ѧʽ��_________��
(3)��T2��1400���¶ȶμ��ȹ���������������ͨ������KMnO4��Һ�У���Һ��ɫ����Ӧ��Ĺ���Ϊ���������H��I�η�����Ӧ�Ļ�ѧ����ʽΪ__________��m=______��
���𰸡� b +5 b ��߶�����̼������Ч�� CaSO4 +CO2+ 2NH3+ H2O=CaCO3+ (NH4)2SO4 �����£�����ص��ܽ�Ƚ�С������ ȡ���һ��ϴ�����õ���Һ������BaCl2��Һ�������� A 17.2g 2 δ�ﵽ��Ӧ������¶� CaSO4H2O 2CaSO4 ![]() 2CaO+2SO2��+O2�� 1.42
2CaO+2SO2��+O2�� 1.42
����������һ����1������˵����һ��ָԪ�أ���ѡb����2���������и�Ԫ�ػ��ϼ۵Ĵ�����Ϊ�㡣��3���ֽ����������ϣ��ֽ���������ڸ��ϲ��ϣ���ѡb����������1����Ӧ������ͨNH3����ͨCO2��Ŀ������߶�����̼������Ч�ʣ� ��ѧ����ʽΪ CaSO4 +CO2+ 2NH3+ H2O=CaCO3+ (NH4)2SO4 ��2�������£�����ص��ܽ�Ƚ�С����������3����Ϊ BaCl2 + CaSO4= CaCl2+ BaSO4����������˵��CaCO3������ϴ�Ӹɾ����¶�Խ�ߣ�K2SO4�ܽ��Խ������������٣���ѡA����������1������Ʒ��CaCO3������Ϊx����ʯ�����ӵ����������ն�����̼��������102.20g-100.00g=2.2g
CaCO3����CaO+CO2��
100 44
x 2.2g
![]() =
=![]() ���x=5g ��CaSO4xH2O��������22.20g-5g =17.2g
���x=5g ��CaSO4xH2O��������22.20g-5g =17.2g
��2��Ũ�������ӵ����������յ�ˮ��������103.60g-100.00g=3.6g
CaSO4xH2O�� CaSO4+xH2O
136+18x 18x
17.2g 3.6g
![]() =
=![]() ���x=2
���x=2
���ģ�(1)0��T1��������û�з����仯��ԭ�������δ�ﵽ��Ӧ������¶ȡ�(2)�����Ѿ������x=2����CaSO4xH2O��CaSO42H2O�������Ȼ���ʧȥ�ᾧˮ�� 3.44g-3.08g=0.36g�� 3.44g-2.72g=0.72g����ͼ�������H��Ĺ��岻���ᾧˮ����G�����Ļ�ѧʽ��CaSO4H2O��(3)��������������ʹ����KMnO4��Һ��ɫ����Ϊ��Ӧ��Ĺ���Ϊ��������������غ㶨�ɣ���Ӧ�Ļ�ѧ����ʽΪ��2CaSO4 ![]() 2CaO+2SO2��+O2�����������⣬M��Ĺ�����CaO��������CaO��������y,2CaSO4
2CaO+2SO2��+O2�����������⣬M��Ĺ�����CaO��������CaO��������y,2CaSO4 ![]() 2CaO+2SO2��+O2��
2CaO+2SO2��+O2��
272 112
3.44g y
![]() =
= ![]() ���y=1.42g
���y=1.42g

 ��У����ϵ�д�
��У����ϵ�д�