��Ŀ����
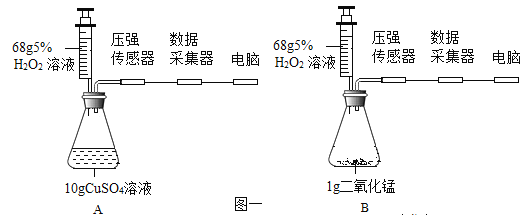
����Ŀ��ij��ѧʵ��С������ͼ��ʾ��ʵ��װ�ý������ʵ�飬����ƿ����װ��Y�Լ���Ȼ��ӽ�ͷ�ι��еμ�x, ��X��������Բ��ƣ������жϲ���ȷ����
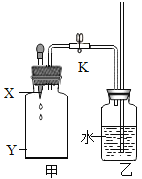
A.��XΪˮ��YΪ����泥�����ƿ���Ҷ˵�����Һ���½�
B.��XΪϡ���ᣬYΪþ������ƿ���Ҷ˵�����Һ������
C.����ƿ���Ҷ˵�����Һ��������YΪˮ����X����Ϊ����������Һ
D.����ƿ���Ҷ˵�����Һ��������YΪ��ʯ�ң���X����Ϊˮ
���𰸡�C
��������
A����XΪˮ��YΪ����泥�����ƿ���Ҷ˵�����Һ���½������������ˮ���¶Ƚ��ͣ���ƿ������������ѹǿ��С���ɼУ���ƿ�Ҷ˵�����Һ���½�����ѡ��A��ȷ��
B����XΪϡ���ᣬYΪþ��ϡ�����þ��Ӧ����������ʹ��ƿ��ѹǿ�����ɼУ���ƿ���Ҷ˵�����Һ����������ѡ��B��ȷ��
C����ƿ���Ҷ˵�����Һ����������ƿ�ڵ��¶������߲������壬ʹ��ƿѹǿ��������������Һ�ε�ˮ��¶Ȳ��ᷢ���ı䣬������������ƹ��������ָ�����ѡ��C����ȷ��
D����ƿ���Ҷ˵�����Һ����������ƿ�ڵ��¶������߲������壬ʹ��ƿѹǿ����ˮ�ε���ʯ����ˮ����ʯ�ҷ�Ӧ�����������ƣ�ͬʱ�ų��������ȣ�ʹ��ƿ���¶����ߣ�����������ͣ���ѡ��D��ȷ��
��ѡ��C��

 �¿α�������������ҵ�������γ�����ϵ�д�
�¿α�������������ҵ�������γ�����ϵ�д� ����ν����Ž̲��㽭���̴�ѧ������ϵ�д�
����ν����Ž̲��㽭���̴�ѧ������ϵ�д�����Ŀ���Ҵ��ڳ��³�ѹ����һ����ȼ���ӷ�����ɫ��Һ�塣�ֽ� 4.6gC2H5OH ��һ���������������ܱ������У�ͨ�������Ҵ���ȫ��Ӧ�������ھ�����ֵ���±������ݱ����ṩ�����ݣ� ����˵���������
C2H5OH | O2 | CO2 | M | H2O | |
��Ӧǰ������g�� | 4.6 | a | 0 | 0 | 0 |
��Ӧ��������g�� | 0 | 0 | 4.4 | b | c |
A.c ����ֵΪ 5.4
B.M ��һ����̼Ԫ��
C.�� a�R9.6 ʱ�������н������� M
D.�� a ��ֵ�ں�����Χ�ڷ����仯ʱ��b��c ��ֵҲ��֮�仯