��Ŀ����
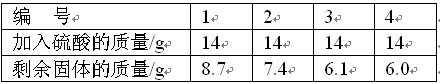
(11��)ij�Ͻ������ͭ��þ��п�е�һ����ɣ������ⶨ����ɣ���������ʵ�飺ȡ�úϽ���Ʒ10.0 g�����ձ��У�Ȼ��56.0 g������������Ϊ14.0%��ϡ����ƽ�����Ĵ����μ�����ձ��У�ÿ�ξ���ַ�Ӧ��ʵ�����ݼ�¼���±���
����
(1)�úϽ���ͭ������������__________��
(2)�úϽ����ͭ�⣬��һ�ֽ�������ʲô�أ�(д������)
(3)�����μ��������ַ�Ӧ��������Һ�����ʵ����������Ƕ��٣�(д��������̣������ȷ��0.1%)

����
(1)�úϽ���ͭ������������__________��
(2)�úϽ����ͭ�⣬��һ�ֽ�������ʲô�أ�(д������)
(3)�����μ��������ַ�Ӧ��������Һ�����ʵ����������Ƕ��٣�(д��������̣������ȷ��0.1%)
(160.0%
(2)��M�������ʵ����ԭ������Ϊx��
M��H2SO4===MSO4��H2��
x 98
10��0 g��6.1 g 42.0 g��14.0%
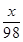 ��
��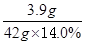
x��65
�ý�����п��
(Ҳ��ѡȡ��һ�λ�ڶ���ʵ������ݼ���)
(3) �⣺�������������Һ��ZnSO4������Ϊy������H2������Ϊz��
Zn��H2SO4===ZnSO4��H2��
98 161 2
42��0 g��14.0% y z
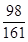 ��
��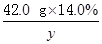
y��9.66 g
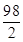 ��
��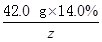
z��0.12 g
ZnSO4��Һ��������������Ϊ
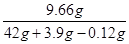 ��100%��21.1%
��100%��21.1%
(Ҳ��ѡȡZn��ʵ�����ݼ���)
���ԡ�
(2)��M�������ʵ����ԭ������Ϊx��
M��H2SO4===MSO4��H2��
x 98
10��0 g��6.1 g 42.0 g��14.0%
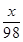 ��
��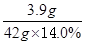
x��65
�ý�����п��
(Ҳ��ѡȡ��һ�λ�ڶ���ʵ������ݼ���)
(3) �⣺�������������Һ��ZnSO4������Ϊy������H2������Ϊz��
Zn��H2SO4===ZnSO4��H2��
98 161 2
42��0 g��14.0% y z
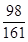 ��
��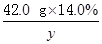
y��9.66 g
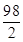 ��
��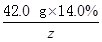
z��0.12 g
ZnSO4��Һ��������������Ϊ
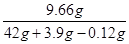 ��100%��21.1%
��100%��21.1%(Ҳ��ѡȡZn��ʵ�����ݼ���)
���ԡ�
��������1�����ݽ�����ԣ�ͭ�������ᷴӦ����4�μ���ϡ�����������������С��ǰ���Σ�˵����ʣ�����ȫ��Ϊͭ��ͭ����Ʒ�����ȿɼ���úϽ���ͭ������������
��2������ǰ���η�Ӧ������������������ɷ�Ӧ�н�������������������ϵ����������������ԭ�����������ݽ��������ԭ�������жϽ�����
��3�����ݷ�Ӧ�Ļ�ѧ����ʽ�������������������������ε��������ų������������������ε������뷴Ӧ����Һ�����ȿɼ���������Һ�����ʵ��������������з�Ӧ����Һ���������������غ㶨�ɼ�����ã�
��𣺽⣺��1��ͭ�������ᷴӦ������ʵ�����ݿ�֪����Ʒ��ͭ������Ϊ6.0g���úϽ���ͭ����������=
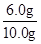 ��100%=60.0%
��100%=60.0%
�ʴ�Ϊ��60.0%��
��2����ȡ�������ε�ʵ������Ϊ����M�������ʵ����ԭ������Ϊx��
�μӷ�Ӧ�Ľ���������=10.0g-6.1g=3.9g�����μӷ�Ӧ��������Һ������Ϊ14g��3=42g��
M+H2SO4�TMSO4+H2��
x 98
3.9g 42.0g��14.0%
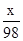 =
=
x="65" ��˸ý�����п��
��Ҳ��ѡȡ��һ�λ�ڶ���ʵ������ݼ��㣩
��3���������������Һ��ZnSO4������Ϊy������H2������Ϊz��
Zn+H2SO4 �TZnSO4+H2��
98 161 2
42.0g��14.0% y z
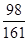 =
=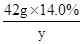
y=9.66g
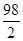 =
=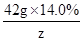
z=0.12g
ZnSO4��Һ��������������=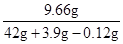 ��100%��21.1%
��100%��21.1%
��Ҳ��ѡȡZn��ʵ�����ݼ��㣩
�𣺣�2���úϽ����ͭ�⣬��һ�ֽ�������п����3�������μ��������ַ�Ӧ��������Һ�����ʵ���������ԼΪ21.1%��
���������������غ㶨�ɣ������μ��������ַ�Ӧ��������Һ������=�μӷ�Ӧ����������+ǰ��������ϡ���������-�ų�����������
��2������ǰ���η�Ӧ������������������ɷ�Ӧ�н�������������������ϵ����������������ԭ�����������ݽ��������ԭ�������жϽ�����
��3�����ݷ�Ӧ�Ļ�ѧ����ʽ�������������������������ε��������ų������������������ε������뷴Ӧ����Һ�����ȿɼ���������Һ�����ʵ��������������з�Ӧ����Һ���������������غ㶨�ɼ�����ã�
��𣺽⣺��1��ͭ�������ᷴӦ������ʵ�����ݿ�֪����Ʒ��ͭ������Ϊ6.0g���úϽ���ͭ����������=
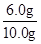 ��100%=60.0%
��100%=60.0%�ʴ�Ϊ��60.0%��
��2����ȡ�������ε�ʵ������Ϊ����M�������ʵ����ԭ������Ϊx��
�μӷ�Ӧ�Ľ���������=10.0g-6.1g=3.9g�����μӷ�Ӧ��������Һ������Ϊ14g��3=42g��
M+H2SO4�TMSO4+H2��
x 98
3.9g 42.0g��14.0%
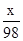 =
=
x="65" ��˸ý�����п��
��Ҳ��ѡȡ��һ�λ�ڶ���ʵ������ݼ��㣩
��3���������������Һ��ZnSO4������Ϊy������H2������Ϊz��
Zn+H2SO4 �TZnSO4+H2��
98 161 2
42.0g��14.0% y z
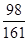 =
=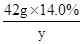
y=9.66g
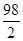 =
=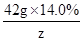
z=0.12g
ZnSO4��Һ��������������=
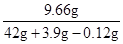 ��100%��21.1%
��100%��21.1%��Ҳ��ѡȡZn��ʵ�����ݼ��㣩
�𣺣�2���úϽ����ͭ�⣬��һ�ֽ�������п����3�������μ��������ַ�Ӧ��������Һ�����ʵ���������ԼΪ21.1%��
���������������غ㶨�ɣ������μ��������ַ�Ӧ��������Һ������=�μӷ�Ӧ����������+ǰ��������ϡ���������-�ų�����������

��ϰ��ϵ�д�
 Сѧ���AB��ϵ�д�
Сѧ���AB��ϵ�д�
�����Ŀ

 Na2SO4 + H2O + CO2����
Na2SO4 + H2O + CO2���� 1g/cm3����Ȼ�������صμ�7.4%�ij���ʯ��ˮ������ȥ100�˳���ʯ��ˮʱ��̼����ǡ�÷�Ӧ��ȫ��
1g/cm3����Ȼ�������صμ�7.4%�ij���ʯ��ˮ������ȥ100�˳���ʯ��ˮʱ��̼����ǡ�÷�Ӧ��ȫ�� ������ȷ��0.01����
������ȷ��0.01����