��Ŀ����
����Ŀ�������ǻ�����������Ҫ����,�������������ʡ�����������ũҩ����ҩ�ȡ���ʷ�������������������Ϊһ�����һ�����������̶ȵı����
Ũ���������ˮ�ԡ���ˮ�Ժ�ǿ�����ԡ�ϡ������ǿ���ԡ�
��ҵ�����������Ϊ:![]()
(1)�ۺϷ������ϲ���,���������֪ʶ,�ش���������:
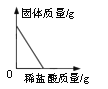
������������ͨ����ԭ�ϻ�����(FeS2)���з���,��Ŀ����___________________��
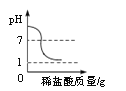
����pH��ֽ��ijһϡ������Һ��pH�IJ�����__________________________��
(2)ij��������������εμ�9.8%��ϡ����ķ����к�һ����������Ʒ�в���Ba(OH)2,���յõ�����ij���23.3g(�������������ʵ�Ӱ��)������ϡ������Һ������___________��
���𰸡� ����Ӧ��ĽӴ�������ӿ췴Ӧ���ʣ����ԭ�ϵ������� �ڰ״ɰ����Ƭ�Ϸ�һСƬpH��ֽ���ò�����պȡϡ����ε�pH��ֽ�ϣ�����ֽ��ʾ����ɫ�����ɫ���Ƚϣ�������ϡ�����pH 100 g
��������(1)������������ͨ����ԭ�ϻ�����(FeS2)���з���,��Ŀ��������Ӧ��ĽӴ�������ӿ췴Ӧ���ʣ����ԭ�ϵ������� ������pH��ֽ��ijһϡ������Һ��pH�IJ������ò�����պȡ���ý�ͷ�ι���ȡ����Һ��������ֽ�ϣ�����һ������ɫ��Ȼ��ͱ���ɫ���Ƚϣ���ȡpH�� (2)�������ϡ������Һ�����ʵ�����Ϊx
Ba(OH)2 + H2SO4 = BaSO4��+ 2H2O
98 233
x 23.3g
![]() x=9.8g
x=9.8g
�����ϡ������Һ������=9.8g��9.8%=100g��
������������Һ������Ϊ100g

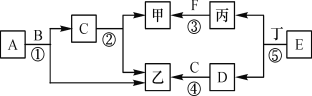
����Ŀ��������ͼװ��̽�����ʵ����������̶ֹ�װ��������

������ a ������___________�� װ�� A �з�Ӧ�Ļ�ѧ����ʽ___________��
��װ�� C ������___________��װ�� D �з�Ӧ�Ļ�ѧ����ʽΪ___________ ��
�� װ�� E �й����ɺ�ɫ�������ɫ����Ӧ��ѧ����ʽΪ___________��
�� װ�� F �ǰ�ȫƿ����������___________��
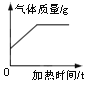
�ݰ�װ�� A �л��ɾƾ��ƣ��������������������̻����ȡ������ �Թ��й���������淴Ӧʱ��仯�����ݼ��±���
��Ӧʱ��/min | 0 | t1 | t2 | t3 |
��������/g | 26.0 | 20.2 | 16.4 | 16.4 |
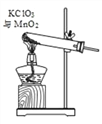
��.���Ƶ�������������_________g��
��.�μӷ�Ӧ������ص����ʵ�����_________mol��ͨ����ѧ����ʽ��ʽ��������

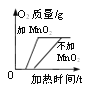
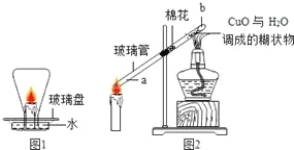
����Ŀ����1��ij��ѧ��ȤС��������ͼ��ʾװ�ý���ʵ�飬���о���ҵ�����Ļ�ѧԭ������Ҫ����գ�

��Ӳ�ʲ������й۲쵽ʲô����__________________
��д��һ����̼����������Ӧ�Ļ�ѧ����ʽ______________________
��ʵ��������ͨ��һ����̼��Ŀ����ʲô________________________
��2��ij��Ӧ���ܱ������н��У���÷�Ӧǰ������ʵ��������,�������ش����⣺
���� | �� | NH3 | CO2 | H2O |
��Ӧǰ����/g | ���� | 0 | 0.1 | 0.2 |
��Ӧ������/g | 0 | 1.7 | 4.5 | 2.0 |
��������һ������__________��Ԫ�أ��÷�Ӧ�Ļ�����Ӧ����________����Ӧ������CO2��H2O��������Ϊ_______�������ʿ�����Ϊ_____���ϣ�Ϊֲ�������ṩ���֣��ٽ�ֲ�ᆬ��Ҷ����ïʢ��ҶɫŨ�̡�
����Ŀ���Ȼ��ơ�̼���Ʋ�ͬ�¶��µ��ܽ�����±���ʾ����ش��������⣺
�¶�/�� | 0 | 10 | 20 | 30 | 40 | |
�ܽ��/g | ̼���� | 7.0 | 12.5 | 21.5 | 39.7 | 49.0 |
�Ȼ��� | 35.7 | 35.8 | 36.0 | 36.3 | 36.6 | |
��1��20��ʱ��30g̼���ƺ�30g�Ȼ��ƵĹ���������뵽100gˮ�У���ֽ����_____����ǡ����й���ʣ�ࡣ
��2���������κ����������ǡ�����ɹ�Σ��Ȼ��ƣ������������̼̼����)��,�䶬���̼����ݵ�ԭ����___________(������ᾧ�����½ᾧ������
��3����300g̼������Һ��150gʯ��ˮ��Һ��Ϻ�,ǡ����ȫ��Ӧ,����,�õ���ɫ����50g�������õ���Һ�����ʵ�������������_____________����