��Ŀ����
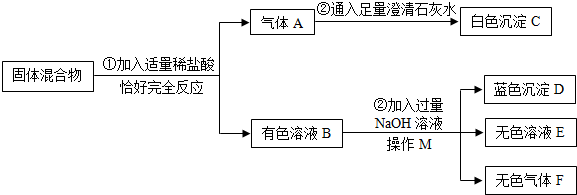
��2013?������һģ��H7N9������������H7N9���������в�������ļ��Ժ�������Ⱦ����Ŀǰ�����ʱȽϵͣ�ΪԤ���������������ӣ�����ClO2���л�����������ȡClO2�ķ�Ӧ����ʾ��ͼ��ͼ1��ʾ��
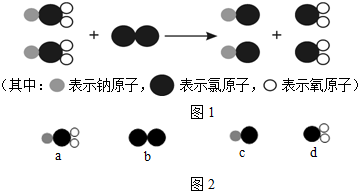
��ش��������⣺
��1����ͼ2��ʾ���������ʣ���a��b��c��d�����������������������
��2��a�������ơ��ȡ���ԭ�ӵĸ�������
��3��d��������Ԫ�غ���Ԫ�����������������Ϊ
��4����ȡClO2�Ļ�ѧ����ʽΪ

��ش��������⣺
��1����ͼ2��ʾ���������ʣ���a��b��c��d�����������������������
d
d
������ĸ������2��a�������ơ��ȡ���ԭ�ӵĸ�������
1��1��2
1��1��2
����3��d��������Ԫ�غ���Ԫ�����������������Ϊ
1��1
1��1
����4����ȡClO2�Ļ�ѧ����ʽΪ
2NaClO2+Cl2=2NaCl+2ClO2
2NaClO2+Cl2=2NaCl+2ClO2
����������1������������Ķ��弰a��b��c��d�������ʵ��۹��ɷ�����������������ʣ�
��2������a���ʵ��۹��ɷ����ơ��ȡ���ԭ�ӵĸ����ȣ�
��3������d���ʵ��۹��ɣ�������Ԫ�غ���Ԫ����������������ȣ�
��4�����ݷ�Ӧ����ʾ��ͼ������Ӧ�������д����Ӧ�ķ���ʽ��
��2������a���ʵ��۹��ɷ����ơ��ȡ���ԭ�ӵĸ����ȣ�
��3������d���ʵ��۹��ɣ�������Ԫ�غ���Ԫ����������������ȣ�
��4�����ݷ�Ӧ����ʾ��ͼ������Ӧ�������д����Ӧ�ķ���ʽ��
����⣺��1����a��b��c��d�������ʵ��۹��ɿ�֪��d����������Ԫ�غ���Ԫ����ɵĻ�������������
��2����a���ʵ��۹��ɿ�֪��a�����к���һ����ԭ�ӡ�һ����ԭ�Ӻ�������ԭ�ӣ���a�������ơ��ȡ���ԭ�ӵĸ�������1��1��2��
��3����d���ʵ��۹��ɿ�֪��d��������һ����ԭ�Ӻ�������ԭ�ӹ��ɣ����ԣ�d��������Ԫ�غ���Ԫ�����������������Ϊ35.5����16��2����1��1��
��4���ɷ�Ӧ�и����ʷ��ӵ���ģ��ͼ�������ʵĻ�ѧʽ�ֱ�ΪNaClO2��Cl2��NaCl��ClO2����Ӧ�Ļ�ѧ����ʽΪ2NaClO2+Cl2=2NaCl+2ClO2��
�ʴ�Ϊ����1��d����2��1��1��2����3��1��1����4��2NaClO2+Cl2=2NaCl+2ClO2��
��2����a���ʵ��۹��ɿ�֪��a�����к���һ����ԭ�ӡ�һ����ԭ�Ӻ�������ԭ�ӣ���a�������ơ��ȡ���ԭ�ӵĸ�������1��1��2��
��3����d���ʵ��۹��ɿ�֪��d��������һ����ԭ�Ӻ�������ԭ�ӹ��ɣ����ԣ�d��������Ԫ�غ���Ԫ�����������������Ϊ35.5����16��2����1��1��
��4���ɷ�Ӧ�и����ʷ��ӵ���ģ��ͼ�������ʵĻ�ѧʽ�ֱ�ΪNaClO2��Cl2��NaCl��ClO2����Ӧ�Ļ�ѧ����ʽΪ2NaClO2+Cl2=2NaCl+2ClO2��
�ʴ�Ϊ����1��d����2��1��1��2����3��1��1����4��2NaClO2+Cl2=2NaCl+2ClO2��
�������������ʵķ��ӹ���ģ��ͼ���жϷ��ӵĹ���д�����ʵĻ�ѧʽ���ǽ�������Ĺؼ���

��ϰ��ϵ�д�
�����Ŀ
 ��2013?������һģ��A��B�������ʵ��ܽ��������ͼ��ʾ��t3��ʱ����A��B�ֱ�����100gˮ�У�������ɱ�����Һ��Ȼ���£��Ը����ܽ�������жϣ�����˵���в���ȷ���ǣ�������
��2013?������һģ��A��B�������ʵ��ܽ��������ͼ��ʾ��t3��ʱ����A��B�ֱ�����100gˮ�У�������ɱ�����Һ��Ȼ���£��Ը����ܽ�������жϣ�����˵���в���ȷ���ǣ�������