��Ŀ����
��7�֣���֪�������ʿ�ʹ��ɫ��ʯ����Һ��ɺ�ɫ��ijͬѧΪ��̽������һЩ���ʣ���������µ�ϵ��ʵ�飺
��1����һС�ձ��м�������ˮ��Ȼ��μ���ɫʯ����Һ����ͼ���۲쵽��ʵ���������ձ����ˮ����� ɫ����ʵ��˵��ˮ�����dz� �����ʡ���ѡ����ԣ����ԣ����ԣ�
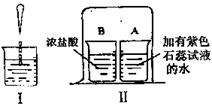
��2�����е��ձ����ټ���ϡ���ᣬ�۲쵽��ʵ������ɫʯ����Һ��� ɫ��
��3���ֱ�ȡA��B�����ձ���A���м�������ˮ���������м�����ɫʯ�B����ʢ��Ũ���ᣬ��һ���ձ���A��B���ձ�����Һ����һ����ͼ����һ��ʱ���۲쵽��������A�ձ��е���ɫʯ����Һ�� ɫ����ʵ��˵���������ʵ��������� ��
��4������ʵ�黹˵��Ũ�����������Ҫ�����ʷֱ��ǣ�
���������ʣ�Ũ������� ��
�ڻ�ѧ���ʣ�Ũ������� �ԡ���ѡ����Ի���ԣ�
��1����һС�ձ��м�������ˮ��Ȼ��μ���ɫʯ����Һ����ͼ���۲쵽��ʵ���������ձ����ˮ����� ɫ����ʵ��˵��ˮ�����dz� �����ʡ���ѡ����ԣ����ԣ����ԣ�

��2�����е��ձ����ټ���ϡ���ᣬ�۲쵽��ʵ������ɫʯ����Һ��� ɫ��
��3���ֱ�ȡA��B�����ձ���A���м�������ˮ���������м�����ɫʯ�B����ʢ��Ũ���ᣬ��һ���ձ���A��B���ձ�����Һ����һ����ͼ����һ��ʱ���۲쵽��������A�ձ��е���ɫʯ����Һ�� ɫ����ʵ��˵���������ʵ��������� ��
��4������ʵ�黹˵��Ũ�����������Ҫ�����ʷֱ��ǣ�
���������ʣ�Ũ������� ��
�ڻ�ѧ���ʣ�Ũ������� �ԡ���ѡ����Ի���ԣ�
�� �� �� ���� �� �� �� ���� �� �� �����˶� ��
�� �� �ӷ� �� �� �� ��
�� �� �ӷ� �� �� �� ��
����������������ʣ����Ǻ�С�ģ����Dz�ͣ�˶��ģ���֮���м�϶��
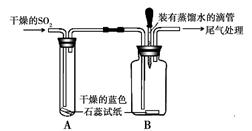
��1����ɫʯ����Һ���������ʱ��ɫ�������������ʱ���ɫ�������������ʲ���ɫ��ˮ��������ɫʯ����Һ��ɫ��˵��ˮ�����Եģ�
��2�����Dz�ͣ�˶��ģ�Ũ������ǿ�ӷ��ԣ��ӷ������Ȼ�����������ˮ��������СҺ�Σ�����������ԣ�������ɫʯ����Һ���ɫ��������A�ձ�����ɫʯ����Һ���ɫ��
���������⿼���֪ʶ��϶࣬�ѶȽϴ�

��ϰ��ϵ�д�
�����Ŀ


 CaCO3��CO2��
CaCO3��CO2��