��Ŀ����
ʳ���Ǽ�ͥ���õĵ�ζƷ��ij��ȤС��ͬѧΪ̽��ʳ���д��ᣨ���ᣩ������������Ʋ�����������ʵ�飺ȡ10%������������Һ���ܶ�Ϊ1��2g/mL����μ��뵽100gʳ���У�������25mL������������Һʱ������ȫ�к͡���֪��Ӧ��ѧ����ʽ���£�
CH3COOH + NaOH �� CH3COONa + H2O���������̽��ʵ���е���ؼ��㣺
��ʵ�������ĵ�25mL����������Һ�����ʵ����ʵ����Ƕ��٣�
��ʳ���д�������������Ƕ���?
��1��25mL��1��2g/mL��10% �� 3g 3/40 = 0��075mol
��2����ʳ���д������������ΪX��
CH3COOH+NaOH=CH3COONa+H2O
60 1 mol
100g��X 0��075mol
X�� 0��045
��ʵ�������ĵ�25mL����������Һ����������Ϊ3g�����ʵ����ʵ���Ϊ0��075mol��ʳ���д��������������0��045��4��5% ��
���������������1��������������Һ���ܶȡ�����������������Լ��������������Һ�������������Ƶ���������Һ���ܶ�=��Һ������/��Һ����������ʵ���������=���ʵ�����/��Һ������*100%�����ʵ����ʵ���=���ʵ�����/�����ʵ�Ħ��������2�����������Ƶ����ʵ�����ʳ������,���ݻ�ѧ����ʽ���Լ����ʳ���д��������������
���㣺���������ʵĻ�ѧ����ʽ���㡢�������������ļ���

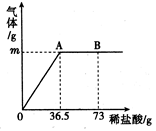
ijʯ��ʯ��Ʒ�ijɷ���CaCO3��SiO2����֪SiO2�Ȳ�����ˮҲ�������ᷴӦ��������С��Ϊ�˲ⶨ��ʯ��ʯ��Ʒ��̼��Ƶ�������������12.0gʯ��ʯ��Ʒ�����ձ��У���ȡ100 gϡ�����4�μ����ձ��У�ÿ�ξ���ַ�Ӧ��ʵ�����ݼ�¼���£�
| �� �� | ��1�� | ��2�� | ��3�� | ��4�� |
| ����ϡ�������� /g | 25 | 25 | 25 | 25 |
| ��Ӧ���ձ������ʵ������� /g | m | 58.4 | 82.6 | 107.6 |
��1�����ɶ�����̼��������������g��
��2����1�μ���ϡ�����ַ�Ӧ���ձ������ʵ�������������g��
��3����ʯ��ʯ��Ʒ��̼��Ƶ�������������д��������̣�

 2CO2+3H2O������100g��������Ϊ92%���Ҵ���Һ�ڿ�������ȫȼ�ղ���������̼������Ϊ���ٿˣ�
2CO2+3H2O������100g��������Ϊ92%���Ҵ���Һ�ڿ�������ȫȼ�ղ���������̼������Ϊ���ٿˣ�  2Fe��3CO2��
2Fe��3CO2��