��Ŀ����
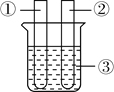
����Ŀ�����ð���(��������������ͬ���Ż��Ϊ40��)̽�������غ㶨�ɵ�ʵ���У�����ͬѧ����Ƥ���ϵ�ϸ�������¶˷ŵ��ƾ��ƻ��������������Ⱥ�Ѹ������Ƥ������ƿ����������ȼ���ף�ʵ���������ͼ��ʾ����ش��������⣺

��1����ʵ���ܷ�������Ϊֱ������Ƥ��ʹ��ƿ�ܷ⣿��˵������____________
��2��ʵ���й۲쵽�����ȱ����С��������֪ʶ���Ͳ����������ԭ����____________
��3����ͬѧ���뵽��ǰ�ⶨ�����������ʱҲ�õ����ף��ҵ�ʱ���ױ�������������ԭ��________������������ͬѧ��Ϊ����̽�������غ㶨�ɵ�ʵ���к���ҲӦ����������Ϊ���Ĺ۵���ȷ�𣿲����Խ���____________________
��4������̽�������غ㶨�ɵ�ʵ���ͬѧΪ����֤��ƿ���Ƿ�������ʣ�࣬�����·�������ƿ������ȼ�ŵ�ľ��������ƿ������ľ������ȼ�գ�˵��ƿ�ڻ�����������ʣ�ࡣ����������ʵ�鷽����ȷ������ͣ�____________________
���𰸡����ܣ����ֱ������Ƥ��ʹ��ƿ�ܷ⣬����ȼ�շų��������ȣ�����ƿ����ѹ�����������ܵ���ƿ���ɳ����Ӷ�����ʵ��ʧ�ܻ�����ȫ�¹ʡ� ����ȼ�շų��������ȣ�����ƿ�����弱�����ͣ���������ȫ��Ӧ��ȴ��ƿ�����������ģ�����ƿ����ѹ��С�������С�� �����ڿ�����ȼ�����������������ĺ�����ʹװ���е�������ȫ��Ӧ ����ȷ��������Ϊ��ʹ���ײ��㣬��Ӧ�İ������������������ɵ���������������Ҳ����ȵģ��ܹ���֤�����غ㶨�ɡ� ����ȷ��������Ϊ��ƿ��ʱ������������ƿ�У�����Ŀ�����ʹľ������ȼ�ա�
��������
��1�����ݰ���ȼ�շų���������ѹ����������������
��2������ƿ����ѹ�ı仯���������
��3�����ݱ��뽫������Ӧ���������ݷ�Ӧǰ�����ʵ�������ȷ��������
��4�����ݴ�ƿ��ʱ������������ƿ�з��������
��1����ʵ�鲻�ܽ������Ϊ��Ƥ�������ֱ������Ƥ��ʹ��ƿ�ܷ⣬����ȼ�շų��������ȣ�����ƿ����ѹ�����������ܵ���ƿ���ɳ����Ӷ�����ʵ��ʧ�ܻ�����ȫ�¹���
��2�������ȱ����С��ԭ����������ȼ�շų��������ȣ�����ƿ�����弱�����ͣ���������ȫ��Ӧ��ȴ��ƿ�����������ģ�����ƿ����ѹ��С�������С��
��3���ⶨ�����������ʱ���ױ��������ԭ�����������ڿ�����ȼ�����������������İ�����ʹװ���е�������ȫ��Ӧ������ȷ��������Ϊ��ʹ���ײ��㣬��Ӧ�İ������������������ɵ���������������Ҳ����ȵģ��ܹ���֤�����غ㶨����
��4������ȷ��������Ϊ��ƿ��ʱ������������ƿ�У�����Ŀ�����ʹľ������ȼ�ա�

 ��������ϵ�д�
��������ϵ�д� ��ӡ�Ļ���ʱ����ϵ�д�
��ӡ�Ļ���ʱ����ϵ�д�����Ŀ������ʦ��ָ���£�ͬѧ�ǽ�������Ȥ�Ļ�ѧʵ��̽����
һ���ⶨ��������������
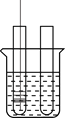
ͼ1��ʾ����С��ͬѧ�ú����ڿ�����ȼ�յIJⶨ�����������ǣ�
��1����������ƿ�ݻ�����Ϊ��ȷݣ������ñ�ǣ�
��2������ȼȼ�ճ��ڵĺ��ף����뼯��ƿ�в�������������
��3����������Ϩ����ȴ���ɼУ�����ˮ�����뼯��ƿ�У����뼯��ƿ��ˮ�����ԼΪ����ƿ���ݻ���1/5����ش��������⣺
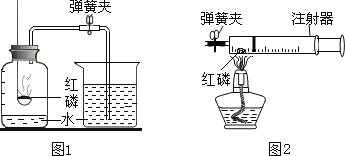
�ٵ�2������ȼ��ʱ������_______________����ѧ��Ӧ����ʽ____________
��ʵ����ϣ������뼯��ƿ��ˮ������������ݻ���1/5������Ϊ������һ�����ԭ�������_________��
A������ƿ�ײ���ˮռ��һ�������
B��������
C������û��ȼ����ͼ��Ŵ���ֹˮ��
��С��ͬѧ��ʵ����з�˼������˸Ľ���������ͼ2��ʾ����С������ʽ��ʼʵ��ǰ���н����ɼУ���ע����������20mL�̶ȴ�����15mL����Ȼ���ɿ��������۲쵽����������20mL�̶ȴ����ò�������ҪĿ����_________������Ϊ�Ľ�����ŵ���_________
����С���Ķ��������ϵ�֪��˫��ˮ�ֽ�����ö������̣�MnO2������������ͭ��CuO����������������������������ͭ������̽����Ȥ��
��������⣩����ͭ�Ƿ�Ҳ��������طֽ�Ĵ��������Ƿ�ȶ������̴�Ч�����ã�
�����ʵ�飩С�������ɵ����������Ϊ�����������������ʵ�飨��������Ӱ��ʵ������ؾ����ԣ�
ʵ����� | ��������� | ������������ | �������� |
�� | 1.2g | / | |
�� | 1.2g | CuO 0.5g | |
�� | 1.2g | MnO20.5g |
������ʵ��Ӧ�� ������������������ʱ��__________��
����ʵ��ڱ�ʵ��ٵ�������������_________��������������С������˵������ͭ�ܼӿ�����صķֽ⣮
�۽�ʵ��ڷ�Ӧʣ��Ĺ���ȡ����ϴ�ӡ�����ٴξ�ȷ�����õ�0.5g��ɫ��ĩ����������Ŀ����________��С��ͬѧ��Ϊ����ͭ�϶�������طֽ�Ĵ�������С��ͬѧ�Դ���������飬С��ͬѧ���������ʵ�飺���ڶ��ξ�ȷ�����õ���0.5g��ɫ��ĩ��1.2g����ػ�Ϸ����Թ��У����ȣ����������ľ��������ľ���ܿ츴ȼ��С��ͬѧ������Ŀ����________��
��Ԥ�ڽ��ۣ�����ͭҲ��������صĴ�����
��������ƣ�����ΪС�����ʵ��ۺ�ʵ��ڶԱȵ�Ŀ����__________�� д��ʵ��ڷ�Ӧ�ı���ʽ______
����Ŀ��С��ͬѧ����±���ʾʵ�飬̽������ͭ��H2O2�ֽ����ʵ�Ӱ�죮
���� | װ�� | ���� |
��1��ȡһ��ͭ˿��������Ͳ�Σ��̶�����˿�ϣ� |
| |
��2���ֱ���ٺ͢���ע��15mL��30%��H2O2��Һ��������ʢ����ˮ�Ģ��С� |
| Լ1min��ɹ۲쵽�ٺ͢��ж����������ݲ����� |
��3����ͭ˿������ͬ�̶�����˿��������С� |
| �����д������ݲ�����5min���������ݲ�������ʱ������Ȼ�������������ݲ����� |
�ش�����������
��1����������ʵ���Ŀ����________��
��2���ܷ�ó�ͭ���Լӿ�H2O2�ֽ����ʵĽ���________����ǡ�����
��3�����������ʵ��̽��ͭ�Dz���H2O2�ֽ�Ĵ�������Ҫ������ʵ�鷽�����в��䣬���б�Ҫ����________��
A������ʵ��ǰͭ˿����������ʵ������ͭ˿��������
B��������������������
C����ʵ����ͭ˿�����м���
D������˿����ͭ˿
E��������������Һ��ˮϡ��
��4��ʵ���ȷ��ͭ���Լӿ�H2O2�ֽ�����ʣ���д���÷�Ӧ�Ļ�ѧʽ����ʽ_______________��