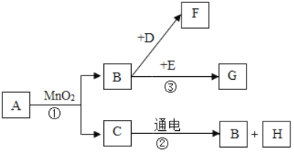
��Ŀ����
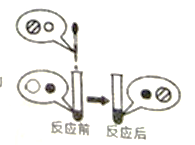
����Ŀ����֪��Cu��ϡ����(H2SO4)����Ӧ��Zn+H2SO4===ZnSO4+H2����ijͬѧ��20g��ͭ(ͭ��п�Ļ����)�����ձ��У����ձ��еμ�ϡ������������ʵ���ü���ϡ�����������������������Ĺ�ϵ����ͼ��ʾ������㣺

(1)ǡ����ȫ��Ӧʱ����������������_____g��
(2)�û�ͭ��п�����������Ƕ��٣�___________
(3)��ǡ����ȫ��Ӧʱ����ϡ���������Ϊ100g����ǡ����ȫ��Ӧʱ�ձ������ʵ�����Ϊ______g��
���𰸡�0.2 32.5% 119.8
��������
��ͭ��ͭ����ϡ���ᷴӦ��
��ͼ��֪��ǡ����ȫ��Ӧʱ��������������Ϊ0.2g��
����뷴Ӧ��п������Ϊx
![]()
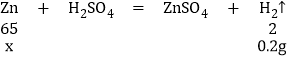
![]() x=6.5g
x=6.5g
����Zn����������=![]()
ǡ����ȫ��Ӧʱ�ձ��ڳ��˲����������ܵ����������ʶ������ձ��У������ձ������ʵ�����Ϊ��20g+100g-0.2g=119.8g��

��ϰ��ϵ�д�
 �����Ƹ���ʦ����ϵ�д�
�����Ƹ���ʦ����ϵ�д� ��ͨ����ͬ����ϰ��ϵ�д�
��ͨ����ͬ����ϰ��ϵ�д�
�����Ŀ