��Ŀ����
����Ŀ��ˮ������֮Դ��
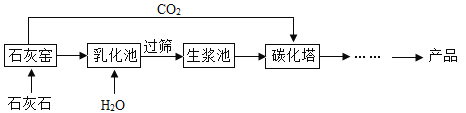
��1��ij��ȤС����ʵ����ģ��ˮ�������ǵĶ���ˮ��������ʵ����������ͼ��ʾ:
��ش���������:
������������������_______________������1��������______________��
������2�ǻ���̿�������˲�����������______________��
��ȡ������Һ��D���Թ��У��������ˮ��,�����н϶�ĸ���������˵����Һ��Ϊ_____________(������ˮ������Ӳˮ��)�������г���________________�ķ�������Ӳˮ��
��2��ˮ�ֽ�ɻ��������������
����ͼ�У��Թ�1������______________(�ѧʽ),��ʵ��֤��ˮ����_____________��ɵġ�
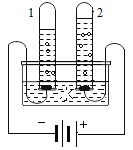
���ҹ�������һ�����ʹ����� ʵ�����ڹ����·ֽ�ˮ����Ӧ�Ļ�ѧ����ʽΪ______________��
���𰸡�����ˮ�е�С���������ٳ��� ���� ����ˮ�е�ɫ�غ���ζ Ӳˮ ��� H2 ��Ԫ�غ���Ԫ�� 2H2O  2H2��+ O2 ��
2H2��+ O2 ��
��������
�������������ɿ���������С�����Ľ�״���������������������ˮ�е�С���������ٳ���������1�ܽ�������Һ����룬����������ǹ��ˣ�
�ڲ���2�ǻ���̿�������˲���������������ˮ�е�ɫ�غ���ζ��
��ȡ������Һ��D���Թ��У��������ˮ��,�����н϶�ĸ���������˵����Һ��ΪӲˮ����Ȼˮ���ʱ��ˮ�п����Ըơ�þ������ת��Ϊ������ˮ��Ӳ�Ƚ��͡������г�����еķ�������Ӳˮ��
��2��ˮͨ��ʱ�����������������������������������Ϊ1��2��
����ͼ�У��Թ�1���Դ�ĸ���������������H2������������Ԫ����ɣ�����������Ԫ����ɣ���ѧ�仯��Ԫ�ص�����䣬��ʵ��֤��ˮ������Ԫ�غ���Ԫ����ɵģ�
���ҹ�������һ�����ʹ����� ʵ�����ڹ����·ֽ�ˮ����Ӧ�Ļ�ѧ����ʽΪ��2H2O  2H2��+ O2 �� ��
2H2��+ O2 �� ��

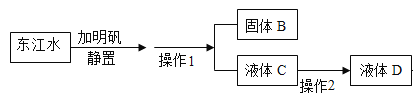
����Ŀ��Cu��Zn�ĺϽ��Ϊ��ͭ���������ĵ����Ժ���ʴ�ԣ��������������������ij��ѧ��ȤС���ͬѧΪ�˲ⶨij��ͭ����ɣ�ȡ20g�û�ͭ��Ʒ���ձ��У���100gϡ�����5�μ������У�ʹ֮��ַ�Ӧ.ÿ������ϡ�����������ʣ�������������¼���ұ�:
����ϡ���������(g) | ��ַ�Ӧ��ʣ�����������(g) | |
��1�� | 20 | 39.9 |
��2�� | 20 | 59.8 |
��3�� | 20 | 79.7 |
��4�� | 20 | 99.6 |
��5�� | 20 | 119.6 |
�Իش���������:
��1����Ӧ����ʱ��������������Ϊ______________��
��2����ͭ��Ʒ��п�����������Ƕ���? (��д���������)______
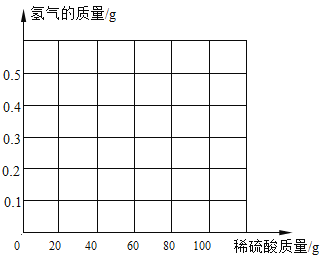
��3��������ͼ���������IJ������ߡ�______
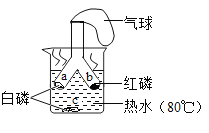
����Ŀ��������û����������ʱ������ˮ��������Ӧ���������£�����ˮ�����ܷ�Ӧ����һ�ֳ��������������һ�����塣С���������ʵ��̽��������ˮ�����췴Ӧ��IJ��

��1���Թ�β����һ��ʪ����Ŀ����_____��
��2��̽�����ɵ�������ʲô��
��ȼ�ŵ�ľ�����������ݣ��б��������Ժ��з���Һ������Ʈ�����С�˵�����ɵ�������_____��
��3��̽���Թ���ʣ�����ɷ���ʲô��
���������ϣ�
�������������� | FeO | Fe2O3 | Fe3O4 |
��ɫ��״̬ | ��ɫ��ĩ | ����ɫ��ĩ | ��ɫ���� |
�ܷ������� | �� | �� | �� |
��ѧ���� | ��������Һ�����������壬������������ͭ��Һ | ||
��������֤���Թ���ʣ�����Ϊ��ɫ����ȫ��������������
����������裩����һ��ʣ�������Fe��Fe3O4���������ʣ�������_____��
��ʵ��̽����
ʵ����� | ʵ�������� |
_____ | _____ |
��ʵ����ۣ�����ˮ������Ӧ�Ļ�ѧ����ʽΪ_____��
����˼�뽻�����ú�ɫ���岻������Fe2O3��������_____��