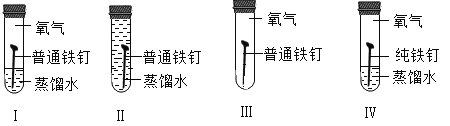
��Ŀ����
����Ŀ����ϸ̼��ƿ�����������Ƭ������Ȳ�Ʒ������̼����������ϸ̼��Ƶ���Ҫ����ʾ��ͼ����:
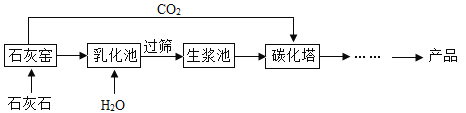
(1)ʯ��ʯ����Ҫ�ɷֵĻ�ѧʽΪ________��ʯ��Ҥ�з����ķ�Ӧ����ʽΪ________��
(2)���黯����,��ʯ����ˮ������Ӧ���÷�Ӧ����________��Ӧ���仯ѧ����ʽΪ______��
(3)��ɸ��Ŀ��������__________(��������������С����)ɸ��ֱ���Ŀ�����
(4)̼�����з�Ӧ�Ļ�ѧ����ʽΪ_______��
���𰸡�CaCO3 CaCO3![]() CaO+CO2�� ���� CaO+H2O=Ca��OH��2 ���� Ca��OH��2+CO2=CaCO3��+H2O
CaO+CO2�� ���� CaO+H2O=Ca��OH��2 ���� Ca��OH��2+CO2=CaCO3��+H2O
��������
��1��ʯ��ʯ����Ҫ�ɷ���̼��ƣ���ѧʽΪCaCO3������ʯ��ʯ���������ƺͶ�����̼����Ӧ�Ļ�ѧ����ʽΪ��CaCO3![]() CaO+CO2����
CaO+CO2����
���CaCO3��CaCO3![]() CaO+CO2����
CaO+CO2����
��2����ʯ����ˮ������Ӧ�����������ƣ��÷�Ӧ���ϡ����һ�������������ڻ��Ϸ�Ӧ��
������ϣ�CaO+H2O=Ca��OH��2��
��3����ɸ��Ŀ�������ش���ɸ��ֱ���Ŀ�����������ڣ�
��4��̼�����ж�����̼���������Ʒ�Ӧ����̼��Ƴ�����ˮ����Ӧ�Ļ�ѧ����ʽΪ��Ca��OH��2+CO2=CaCO3��+H2O�����Ca��OH��2+CO2=CaCO3��+H2O��