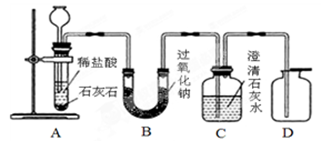
��Ŀ����
����ά���������������������Ԫ�ء��ƶ����dz��õIJ��Ƽ�����Ҫ�ɷ���CaCO3���ƶ���ÿƬ2.0g��ȡ1Ƭ�ƶ��棬����ʢ��10gϡ������ձ��У�����̼��Ƹ�����ǡ����ȫ��Ӧ�������ɷ��������Ӧ�����ձ�������������Ϊ11.34g���Լ��㣺
��1����Ӧ����������̼ g���� mol��
��2��ÿƬ�ƶ����к�̼��� mol��
��3�����øƶ���ͨ��һ��2�Σ�ÿ��1Ƭ����ÿ��ÿ�������Ԫ�ص�����Ϊ g��
��4�����ݻ�ѧ����ʽ��ʽ����������Ӧ����ϡ�������ʵ�������������ȷ��0.01%��
��1��0.66 0.015 ��2��0.015 ��3��1.2 ��4��10.95% ��1�֣�
���������������1���������غ㶨�ɿ�֪����Ӧǰ���ʵ����������ڷ�Ӧ�����ʵ������������ɶ�����̼������Ϊ��2g+10g-11.34g="0.66" g
0.66 g��Ħ������0.66 g��44g/ mol="0.015" mol
��2���ɻ�ѧ����ʽCaCO3 +2HCl ��CaCl2 +H2O + CO2����֪��ÿƬ�ƶ����к�̼���̼���0.015mol
��3��ÿ��ÿ�������Ԫ�ص�����Ϊ2��0.015��100��40��=1.2g
��4���裺�������Ȼ���Ϊx mol
CaCO3 +2HCl ��CaCl2 +H2O + CO2����1�֣���ѧʽ��ȷ�͵÷֣�
1 2
0.015 x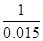 =
=  ��1�֣� x =" 0.03" mol ��1�֣�
��1�֣� x =" 0.03" mol ��1�֣�
�������������=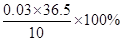 = 10.95%
= 10.95%
���㣺���ʵ����������غ㶨�ɣ����ݻ�ѧ����ʽ�ļ��㡣

 ��У����ϵ�д�
��У����ϵ�д���ϡ���������ͭ�Ļ����Һ�У������������ۣ�ʹ��ǡ�÷�Ӧ����.��ԭ�����Һ�����������ͭ��������Ϊ
| A��7:80 | B��1:1 | C��7:40 | D��49:80 |