题目内容
某兴趣小组同学将CO2分别通入澄清石灰水和氢氧化钠溶液中,观察到前者变浑浊,后者无明显现象。
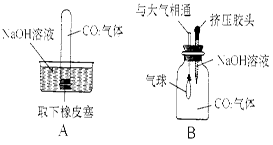
(1)写出二氧化碳与氢氧化钠溶液发生反应的化学方程式: 。
(2)为探究CO2和NaOH是否发生了化学反应,王强设计了如图所示的A、B实验,验证CO2与NaOH溶液发生了化学反应。实验现象为:A中试管内液面上升;B中气球胀大。
①王强认为上述实验是可行的,其共同原理是
。
②李莉提出质疑,她认为上述实验不严谨,其理由是 。要得到科学严谨的结论,仍利用该装置,补做的对比实验是 。
③杨红通过检验生成物:向B实验后的溶液中加入 ,观察到 现象,从而也证明CO2与NaOH发生了反应。
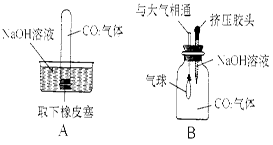
(1)写出二氧化碳与氢氧化钠溶液发生反应的化学方程式: 。
(2)为探究CO2和NaOH是否发生了化学反应,王强设计了如图所示的A、B实验,验证CO2与NaOH溶液发生了化学反应。实验现象为:A中试管内液面上升;B中气球胀大。
①王强认为上述实验是可行的,其共同原理是
。
②李莉提出质疑,她认为上述实验不严谨,其理由是 。要得到科学严谨的结论,仍利用该装置,补做的对比实验是 。
③杨红通过检验生成物:向B实验后的溶液中加入 ,观察到 现象,从而也证明CO2与NaOH发生了反应。
(6分)(1)CO2+2NaOH=Na2CO3+H2O
(2)①氢氧化钠溶液与CO2发生反应,使容器内气压降低
②CO2能溶于水,也能使容器内气压降低 将氢氧化钠溶液换成等体积的水
③稀盐酸(或氯化钙溶液) 有气泡产生(或有沉淀生成) (本小题合理答案均可)
(2)①氢氧化钠溶液与CO2发生反应,使容器内气压降低
②CO2能溶于水,也能使容器内气压降低 将氢氧化钠溶液换成等体积的水
③稀盐酸(或氯化钙溶液) 有气泡产生(或有沉淀生成) (本小题合理答案均可)
:(1)根据反应物和生成物即可写出反应方程式为:CO2+2NaOH=Na2CO3+H2O
(2)①氢氧化钠溶液吸收了二氧化碳后,锥形瓶中的压强减小,在外界大气压的作用下小气球胀大;
②二氧化碳不但能与氢氧化钠反应还能与水反应,不能证明二氧化碳与碳酸钠发生了反应;
故为了更严谨,应将氢氧化钠溶液换成等体积的水来与A实验对比.
③因为二氧化碳与氢氧化钠反应会生成碳酸钠,要验证二氧化碳是否与氢氧化钠发生了反应,就要验证是否有碳酸根,故可向B实验后的溶液中加热稀盐酸,观察到有气泡产生,就可以证明二氧化碳与氢氧化钠发生了反应.
(2)①氢氧化钠溶液吸收了二氧化碳后,锥形瓶中的压强减小,在外界大气压的作用下小气球胀大;
②二氧化碳不但能与氢氧化钠反应还能与水反应,不能证明二氧化碳与碳酸钠发生了反应;
故为了更严谨,应将氢氧化钠溶液换成等体积的水来与A实验对比.
③因为二氧化碳与氢氧化钠反应会生成碳酸钠,要验证二氧化碳是否与氢氧化钠发生了反应,就要验证是否有碳酸根,故可向B实验后的溶液中加热稀盐酸,观察到有气泡产生,就可以证明二氧化碳与氢氧化钠发生了反应.

练习册系列答案
相关题目