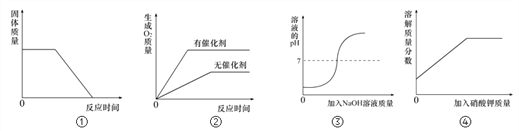
��Ŀ����
����Ŀ��Ԫ�����ڱ���ѧϰ���о���ѧ����Ҫ���ߡ���ͼ��Ԫ�����ڱ���һ���֡�

(1)ѧϰ��ѧ������ѧ���˴��۽Ƕ���ʶ���
���������������ӵĽṹʾ��ͼ���ش����⡣
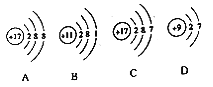
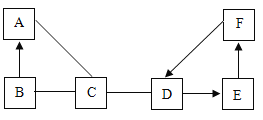
����������ͬ��Ԫ�ص���______(����ţ���ͬ)��
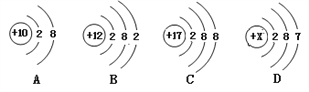
��ͼC��ijԪ�ص�ԭ�ӽṹʾ��ͼ����Ԫ����Ԫ�����ڱ��е�λ����______(ѡ��ٻ�ڻ��)��
(2)ԭ������Ϊ12��Ԫ�ص�ԭ���ڻ�ѧ��Ӧ����_______(������������ʧ��)���γ����ӷ�����_______����Ԫ���ڻ������еĻ��ϼ�Ϊ_____�ۣ���Ԫ�غ�ԭ������Ϊ9��Ԫ����ɵĻ�������________(�ѧʽ)��
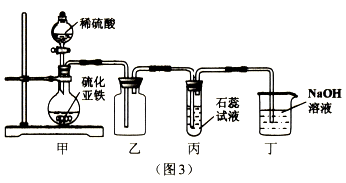
(3)̽����������ˮ��Ӧʱ���������������ɣ��ռ����岢��ȼ�������Ա���������Ӧ����Һ�еμӷ�̪�Լ�����Һ�ʺ�ɫ���ɴ��ƶϣ�����ˮ��Ӧ�Ļ�ѧ����ʽΪ ____________________��
��������Ϣ��������ʵĽṹ������֮��Ĺ�ϵ��ʲô��ʶ��_______________________��
���𰸡� AC �� ʧ Mg2+ +2 MgF2 2Na+2H2O=2NaOH+H2�� �ṹ�������ʣ����ʷ�ӳ�ṹ
�����������⿼����Ԫ�����ڱ����ص㼰��Ӧ����ԭ�ӽṹʾ��ͼ�����ӽṹʾ��ͼ���й�Ԫ�ػ��ϼ۵ļ�������ѧ����ʽ����д���ѶȲ��������е�֪ʶ���з����жϡ�
��1����������ͬ��������һ��ԭ���ܳ�ΪԪ����AC�еĺ�����������ȣ���������ͬ��Ԫ�أ�
��ͼC��ijԪ�ص�ԭ�ӽṹʾ��ͼ����Ԫ�غ�����������17������16��Ԫ��֮����˸�Ԫ����Ԫ�����ڱ��е�λ���Ǣۣ�
��2��ԭ������Ϊ12��Ԫ����þԪ�أ�þԭ��������������2���ڻ�ѧ��Ӧ����ʧ���ӣ��γɴ�2����λ����ɵ����ӣ��������Mg2+��þԪ���ڻ������еĻ��ϼ�Ϊ+2�ۣ�þԪ�غ�ԭ������Ϊ9��Ԫ����ɵĻ������У���Ԫ�ػ��ϼ���-1����������Ԫ�ػ��ϼ۴�����Ϊ�㣬�γɵĻ�����Ļ�ѧʽ��MgF2��
��3��̽����������ˮ��Ӧʱ���������������ɣ��ռ����岢��ȼ�������Ա���������Ӧ����Һ�еμӷ�̪�Լ�����Һ�ʺ�ɫ���ɴ��ƶϣ�����ˮ��Ӧ�����������������ƣ���Ӧ�Ļ�ѧ����ʽΪ��2Na+2H2O=2NaOH+H2������������Ϣ��֪���ṹ�������ʣ����ʷ�ӳ�ṹ��