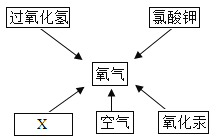
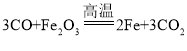��Ŀ����
����Ŀ��ijƷ�ƴ����к��������Ȼ��ơ�ij��ѧ̽��С�����ⶨ��Ʒ�ƴ���Ĵ��ȣ���̼���Ƶ�������������
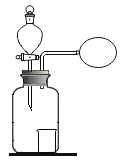
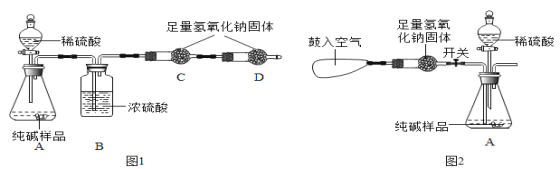
��һ������ͬѧ�����ͼ1��ʾʵ�飺
��ʵ�鲽�裩
����װ����ͼ�����������װ�õ������ԣ�
�ڳ�ȡ13.0g��Ʒ������ƿ�У�������������ˮ�ܽ⣬��������װ���м�����Ӧ��ҩƷ��
�۳���װ��C��������
�ܴ�Һ©����������ϡ���ᣬֱ�����ٲ�������Ϊֹ��
���ٴγ���װ��C����������
�������Ʒ��̼���Ƶ�����������
��ʵ�������
��1��Aװ���з�Ӧ�Ļ�ѧ����ʽΪ_____��
��2��Bװ�õ�������_____��
��3�����û��Bװ����������Ʒ��̼���Ƶ���������_____����ƫ��ƫС�����䣩��
��4��С������ܷ�������������ͬѧ�Ǿ���������Ϊ���ܣ�������_____��
��5��������ʵ�鲽�裬ʵ��ǰ����Cװ�ã�����ҩƷ���������ֱ�Ϊ61.2g��65.6g����ô�����Ʒ�Ĵ���Ϊ_____%����ȷ��0.1%����
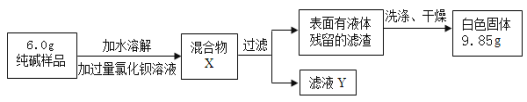
����������ͬѧ�������ɳ����ķ������ⶨ��Ʒ�д�����������������������ʵ�飺
��1���жϼ����Ȼ�����Һ�Ƿ�����ĺ��ʷ�����_____��Ȼ��۲������жϡ�
A ���û����X�����ϲ���Һ�м����μ��Ȼ�����Һ�����ް�ɫ�������ɣ����Ȼ����Ѿ�������
B ������X�еμ�ϡ���ᣬ���а�ɫ����������˵���Ȼ����Ѿ�������
��2���ж������Ƿ�ϴ�Ӹɾ������Բ�ȡ������ϴ��Һ�еμ�_____��Ȼ��۲������жϡ�
A �Ȼ�����Һ B ϡ���� C ��������Һ D ϡ����
��3������ʵ�����ݣ���������Ʒ��̼���Ƶ���������Ϊ_____%��д�����������̣������ȷ��0.1%����
��ʵ�鷴˼��
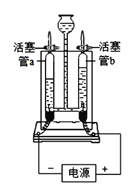
����ͬѧ��Ϊ��ʹ��ͼ1װ�û�ʹ��õ�̼���Ƶ���������_____������ƫ��������ƫС�����������ж��� ��Ϊ�˿˷�ͼ1װ�õIJ��㣬��ͬѧ�����Aװ������ͼ2�ĸĽ���Aװ����ԭ˫�����������������������ڷ�Ӧǰ������ͼ2װ�÷����λ��������������Ӧ����ͼ2װ�ù��������������_____��
���𰸡�Na2CO3+H2SO4=Na2SO4+H2O+CO2�� ��ȥˮ���� ƫ�� ����ӷ����Ȼ������屻װ��C���գ�Ӱ��ʵ��ⶨ 81.5 A C 88.3 ƫС ��������װ���ڵĶ�����̼ȫ���ų�
��������
[ʵ�����]
��1��̼���ƺ����ᷴӦ���������ơ�ˮ�Ͷ�����̼��Aװ���з�Ӧ�Ļ�ѧ����ʽΪNa2CO3+H2SO4=Na2SO4+H2O+CO2����
��2��Ũ���������ˮ�ԣ�����Bװ�õ������dz�ȥˮ������
��3��Cװ�������ӵ������������ɵĶ�����̼�����������û��Bװ�ã���ʹ������ɶ�����̼��������������̼���Ƶ�����Ҳ���࣬��û��Bװ����������Ʒ��̼���Ƶ���������ƫ��
��4������ӷ����Ȼ������屻װ��C���գ�Ӱ��ʵ��ⶨ�����Բ���������������
��5������Ʒ��̼���Ƶ�����Ϊx��
������̼������=65.6g-61.2g=4.4g
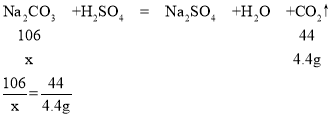
x=10.6g
��Ʒ��̼���Ƶ���������Ϊ ![]() ��100%=81.5%
��100%=81.5%
����Ʒ��̼���Ƶ���������Ϊ81.5%��
��������1���Ȼ�����Һ��̼���Ʒ�Ӧ����̼�ᱵ�����������жϼ����Ȼ�����Һ�Ƿ�����ĺ��ʷ����Ǿ��û����X�����ϲ���Һ�м����μ��Ȼ�����Һ�����ް�ɫ�������ɣ����Ȼ����Ѿ�������
��2���Ȼ��ƺ���������Һ��Ӧ�����Ȼ�����ɫ�����������ж������Ƿ�ϴ�Ӹɾ������Բ�ȡ������ϴ��Һ�еμ���������Һ��
��3����ԭ�������̼���Ƶ�������y
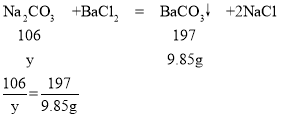
y=5.3g
��������Ʒ��̼���Ƶ���������Ϊ ![]() ��100%=88.3%
��100%=88.3%
����������Ʒ��̼���Ƶ���������Ϊ88.3%��
[ʵ�鷴˼]
��������������ɵĶ�����̼��������ƿ�У�δ��װ��C���գ�Ӱ��ʵ��ⶨ�����Լ�������ͬѧ�����Ʒ����̼���Ƶ����������������ȷ���IJⶨ���ƫС����Ӧ����ͼ2װ�ù�������������ǽ�������װ���ڵĶ�����̼ȫ���ų���

 ��������ϵ�д�
��������ϵ�д�����Ŀ��ij�����ڵ�ȼ�������·�����Ӧ��������ͷ�Ӧ�ﹲ���֣����ǵ���ʾ��ͼ�ͷ�Ӧǰ����������±���ʾ��
������� | �� | �� | �� | �� |
|
��ʾ��ͼ |
|
|
|
| |
��Ӧǰ����/g | 68 | 100 | 1 | 0 | |
��Ӧ������/g | 0 | x | y | z |
��1�����е����������У��������������______ (�ѧʽ����
��2��������Ӧ�Ļ�ѧ����ʽΪ______��
��3��һλͬѧ�ڼ���x��y��z��ֵ�Ĺ����У��г������µ�ʽ��������ȷ����______������ĸ��ţ���
A x + y +z = 169 B y + z = 168 C (100-x)��z = 32��64 D (l00-x)��(y-1) = 8��3