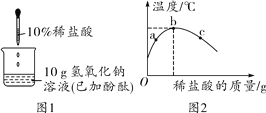
��Ŀ����
����Ŀ��������̼��һ����������壬��ʵ���ҿ��Ժܷ������ȡ��
������һ��ѡȡҩƷ�뷴Ӧװ�á�
ʵ���Ҿ���ѡ��ϡ�����ʯ��ʯ��Ϊ��Ӧ����ȡCO2���壬������Ӧ�Ļ�ѧ����ʽΪ��_________��
����������������������װ�������ԡ�
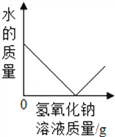
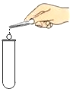
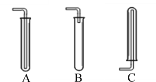
��1�������й�������������������������ȷ����___
A ����������Լ1/2�����Թܿ�
B �齺���벣����������ǰ����ˮ��ʪ�����ܿ�
C �齺��һ����������������
D �������ܿ�һ�������齺����
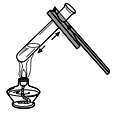
��2����װ����������ͼ������ĩ�˽���ˮ�У�������ס�Թܣ����ֵ��ܿ�____�������������á�
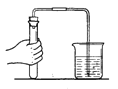
������������Ӧ���ռ����塣
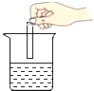
��С�Թ��У�����ˮ���������ſ��������ռ�һ�Թ�CO2��
��1�����ſ������ռ�CO2��Ӧѡ����ͼ�е�______װ�á�
��2������ˮ���ռ�CO2���ж������Ѽ����������ǣ�_________��
�������ģ����������̼���塣
![]()
��ͼ��ʾ������CO2���Թ��ڵ���Լռ�Թ��ݻ�����֮һ�ij���ʯ��ˮ�������������ϡ��³�����һ��ʱ�䡣
��1��д��ʵ���Ҽ���CO2�Ļ�ѧ����ʽ___________��
��2��������Թ���ѹǿ____�Թ������ѹǿ��ѡ����>������=������<������
��3�����Թ�ʱ���Թܿ������Ͻ�������Ŀ���ǣ���_________�� �� ________��
���𰸡�CaCO3+2HCl�TCaCl2+H2O+CO2�� C ������ð�� A ����ƿ��������ð�� Ca��OH��2+CO2=CaCO3��+H2O �� ��ֹʯ��ˮ����Թܸ�ʴƤ�� �ٽ�ʯ��ˮ������ն�����̼
��������
[����һ]̼��ƺ����ᷴӦ�����Ȼ��ơ�ˮ�Ͷ�����̼����ѧ����ʽΪ��CaCO3+2HCl�TCaCl2+H2O+CO2����
[�����]��1��A���������������Թܿڣ������²����������𣬹���ȷ��
B���齺���벣����������ǰ��ˮ��ʪ�ܿڣ��������ã�����ȷ��
C���������ܿ�һ�������齺��һ�ˣ���C����
D���������ܿ�һ�������齺��һ�ˣ�����ȷ��
��ѡ��C��
��2����װ����������ͼ1����ĩ�˽���ˮ�У�������ס�Թܣ����ֵ��ܿ�������ð���������������ã�
[������]��1��������̼���ܶȱȿ�����Ӧ���������ſ������ռ�������Ӧ�������Թܵײ�����ѡA��
��2��������̼���ܶȱ�ˮС����������ˮ���ռ�CO2���ж������Ѽ����������ǣ�����ƿ��������ð����
[������]��1��������̼���������Ʒ�Ӧ����̼��Ƴ�����ˮ����ѧ����ʽΪ��Ca��OH��2+CO2=CaCO3��+H2O��
��2��������̼���������Ʒ�Ӧ�����Թ��ڵ�ѹǿ��С�����Գ�����Թ���ѹǿ���Թ������ѹǿ��
��3�����Թ�ǰ������������Ŀ���ǣ���ֹʯ��ˮ����Թܸ�ʴƤ�����ٽ�ʯ��ˮ������ն�����̼��
�ʴ�Ϊ��[����һ]CaCO3+2HCl�TCaCl2+H2O+CO2����
[�����]��1��C��
��2����������
[������]��1��A��
��2������ƿ��������ð����
[������]��1��Ca��OH��2+CO2=CaCO3��+H2O��
��2������
��3����ֹʯ��ˮ����Թܸ�ʴƤ�����ٽ�ʯ��ˮ������ն�����̼��

����Ŀ����ѧϰ�ε�����ʱ��ij��ͬѧ��̼������Һ��һƿ��ǩ����ij��л�ѧ������ҺM����ͼ�����з�Ӧ���а�ɫ�������������˺�õ�������Һ��ͬѧ�ǶԸ�ʵ�������һϵ�е�̽����
̽��һ��M��Һ�е�������ʲô��
��������룩����ͬѧ��ΪM��Һ�����ʿ�����Ca(OH)2��_______��
�����ʵ�飩ͬѧ����������·���������ʵ�飺
ȡ����M��Һ���Թ��У�������________________��
���ۣ�ԭ��Һ�е�������Ca(OH)2��
̽������M��̼������Һ��Ӧ���˺������Һ�л�����Щ���ʣ�
��������룩
ѧ���ײ��룺NaOH��Na2CO3��ѧ���Ҳ��룺_________ ��ѧ�������룺NaOH��
��ʵ����֤����ͬѧ����Լ��������������ʵ�飺
ʵ����� | ʵ������ | ʵ����� |
ȡ�����Թ��У����뼸��ϡ���� | û�����ݲ��� | �Լ����벻������ѧ�����IJ�����ȷ |
��ͬѧ��ʵ������Ƿ���ȷ��Ϊʲô��_________________________��
������̽����Ϊ��֤���Լ�������ȷ����ͬѧ��Ƶļ�ʵ������ǣ��ֱ�ȡ������Һ������һ����Һ��_________������һ����Һ��__________��
���ܽύ����ͬѧ����ʶ��ʵ����һ��Ҫע�Ᵽ���Լ�ƿ�ı�ǩ��
����Ŀ������ͭ��һ��Ӧ�ü���㷺�Ļ���ԭ����������ˮ���������ھƾ���ͭ������ȫ������pH��5���ң���������ȫ������pH��2����.������ij�����ú�����ͭΪԭ������������CuSO4��5H2O��������ʯ�ࣨCaSO4��2H2O������������ʾ��ͼ��
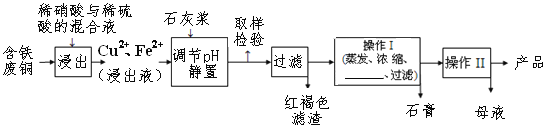
������ʯ���ڲ�ͬ�¶��µ��ܽ�ȣ�g/100gˮ�������
�¶�(��) | 20 | 40 | 60 | 80 | 100 |
ʯ�� | 0.32 | 0.26 | 0.15 | 0.11 | 0.07 |
���� | 32 | 44.6 | 61.8 | 83.8 | 114 |
��ش��������⣺
��1�����ɫ��������Ҫ�ɷ���____________��
��2��ʯ�ҽ���pH��ԼҪ���ڵ�__________
A. 2 B. 5 C. 7 D.10
��3�������ķ�ͭ��ϡ���ᷴӦ�Ļ�ѧ����ʽ________________________________��
��4������I����¶�Ӧ�ÿ�����___________�����ң�
��5������Һ�з��������ͭ����IJ�����ӦΪ����Ũ����__________�����ˡ�ϴ�ӡ������������ˮ�Ҵ���ϴ��Һ����������ˮ��ԭ����_________________��