��Ŀ����
����Ŀ�����ѵĹ����Ƥ�ж����зḻ��Ӫ�����ʡ�
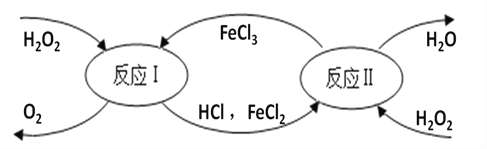
��1��Ϊʹ���ѹ�ʵ��������ߺ��������������������к��ڿ��ʵ�ʩ������ء�������Ƶȷ��ϣ�������������ڻ�ѧ�����е�___________�ʡ�
��2��������ͭ���Ƶ�ũҩ������Һ�����Է������������еIJ������ܽ�����ͭʱ�����������������û�ѧ����ʽ��ʾ��ԭ��___________________��
��3��ʳ������ǰҪ��ϴ�ɾ�����ͼ��һ����ϴ�ķ�����
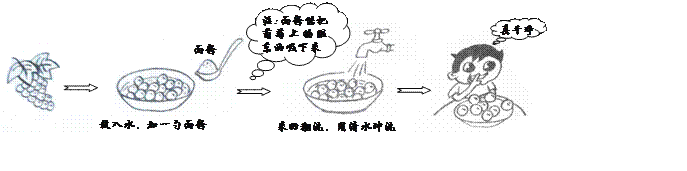
����˵����ȷ����_______(�����)��
A. ���������ȥ������Ƥ�ϵ��ණ��
B. ��ϴ�ɾ�����������µ�����Ƥ
C. ���Ѹ����������ʣ������˲���ʳ
���𰸡� �� Fe + CuSO4 = FeSO4 + Cu AC
�����������⿼���˳������ʵ����࣬�����Ļ�ѧ���ʵȡ��ѶȲ�����ϸ�������н��
��1��������к��м�Ԫ�����ڻ�ѧ�����еļطʣ�
��2����������ͭ��Ӧ��������������ͭ����ѧ����ʽ��Fe + CuSO4 = FeSO4 + Cu��
��3��A������ܽ������ϵ��ණ�������������������ȥ������Ƥ�ϵ��ණ������ȷ��
B�����ѵĹ����Ƥ�ж����зḻ��Ӫ�����ʣ��������µ�����Ƥ��һ���˷ѣ�����C�����Ѹ����������ʣ������˲��˶�ʳ����ȷ����ѡAC��

 �п������п��Ծ����ϵ�д�
�п������п��Ծ����ϵ�д� ��������״Ԫ��ϵ�д�
��������״Ԫ��ϵ�д� �ƸԿ�����ҵ��ϵ�д�
�ƸԿ�����ҵ��ϵ�д�����Ŀ��СӢ��С����ѧ����Ļ�ѧ���ʺ���������ͼ1�Ģ�--�ݸ�ʵ�飬

��ͼ1�ش��������⣺
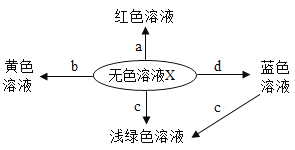
(1)��Ӧ���Թܢڢ���һ�����е������ӷֱ���_______��_______(�����ӷ���)��
(2)ʵ����ڷ�Ӧʱû�����Ե�ʵ��������β���ȷ��ʵ��ܷ�Ӧ�ķ���_______��
(3)д��ʵ��ݷ�����Ӧ�Ļ�ѧ����ʽ__________�������ӵĽǶȷ������˻�ѧ��Ӧ�ܷ�����ԭ�� _________��ʵ�������СӢ���ν��Թܢܢݵķ�Һ��������ͬһ�ྻ�ķ�Һ����(����ͼ2)���۲쵽��Һ���������ݲ��������а�ɫ�������ɡ�ͨ����Һ���г�������������ȷ�����Թܢܢ��з�Һ���е����ʷֱ��ǣ��Թܢ� ___________ ���Թܢ�________________��
(4)СӢ����Һ�������ʽ��й��ˣ��õ���ɫ��������ɫ��Һ����̽����ɫ��Һ�����ʵijɷ֡�СӢ�����ó���ɫ��Һ��һ������HCl�������� _____________________��
��������⣩���˳�����ɫ��Һ�к���ʲô���ʣ�
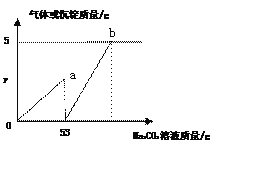
���������룩 ��NaCl�� ��NaCl��CaCl2�� ��NaCl ��CaCl2��Na2CO3�� ��NaCl��Na2CO3��С����Ϊ����_________һ������ȷ��
������ʵ�飩
ʵ�鲽�� | ʵ������ | ʵ����� |
СӢȡ������Һ���Թ��У��μ�����̼������Һ | ���������� | ����2������ |
СӢȡ������Һ���Թ��У��μ�����_____��Һ | �г������� | ����______ ���� |
�������뽻������λͬѧȷ�������շ�Һ�����ʵijɷ֡�����Ϊ�����÷�Һ�ķ�����________��
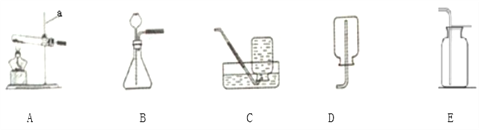
����Ŀ����ͼ��С��ͬѧ��Ƶ�һ��ʵ�����Ʊ������������CO2����֤CO2����NaOH��Ӧ��װ�á�
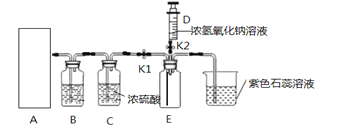
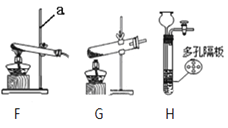
(1)д������a�����ƣ�______________��
(2)��ʵ�����У�Ϊ�Ƶö�����̼��Ӧѡ��װ��______����A����
(3)Ϊ��ô����Ķ�����̼��װ��B��Ӧ��_____��װ��C��Ũ�����������___________��
a.NaOH��Һ b.����ʯ��ˮ c.���͵�NaHCO3��Һ d.Ũ����
(4)��װ��E���ռ���CO2ʱ���ر�ֹˮ��K1����ֹˮ��K2����ע����D��5mLŨNaOH��Һѹ��װ��E�У�������Ӧ�Ļ�ѧ����ʽΪ_______________���۲쵽��������______________��Сƽͬѧ��ΪҪ֤��CO2��NaOHȷʵ��Ӧ����Ӧ��ע����NaOH��Һ���ɵ������_______������һ��ʵ�顣
(5)С����Ϊ�����ǻ����Բ��ü���������ķ�����ȷ��CO2��NaOH��Ӧ�ˡ����ǣ�С��ͬѧȡ����E����Һ���ձ��У�������Һ�еμӼ���ϡ���ᣬδ���������ݲ������ݴ�����С����ΪCO2��NaOHû�з�Ӧ������Ϊ�ý����Ƿ���ȷ����˵�����ɡ�__________________��
(6)Ϊ�˽�һ���ó����ۣ���λͬѧ�����ѧ֪ʶ�ֹ���������˼���棬���������ʵ�������֤���뽫�±�����������
ʵ�鲽�� | ʵ������ |
��ȡE����Һ���μӹ���CaCl2��Һ | _____________________________ |
�ڶԢٽ��й��ˣ�����Һ�еμ�_________ | ��Һ��� |
�����ۡ�CO2��NaOH�ܷ�Ӧ���ҷ�Ӧ�����Һ�л���NaOH��
����˼���ۡ�
(1)��ʵ�鲽�����ΪʲôҪ���������CaCl2��Һ��_____________________________��
(2)С��ͬѧ��Ϊ��ʵ�鲽����п�����Ca(OH)2 ��Һ����CaCl2֤����һƿ���Ƿ���NaOH������Ϊ�أ���˵������______________________________________________��