��Ŀ����
��2011?�����֪��RCH2COOH

 +RCl��
+RCl�� +NaCl
+NaCl
I������ƽF�ǽ�Ѫ֬�������̴���ҩ�����һ���ϳ�·�����£�

��1��AΪһԪ���ᣬ8.8gA������NaHCO3��Һ��Ӧ����2.24L CO2����״������A�ķ���ʽΪ
��2��д������A����ʽ�����м������Ľṹ��ʽ��
 ��
��
��3��B���ȴ����ᣬ��˴Ź��������������壬д��B��C�ķ�Ӧ����ʽ��
 ��
��
��4��C+E��F�ķ�Ӧ����Ϊ
��5��д��A��F�Ľṹ��ʽ��A��
 ��F��
��F��
 ��
��
��6��D�ı������������⣬�����������ŵ�����Ϊ
II��������·�ߣ���C�ɺϳɸ߾���H��
C
G
H
��7��C��G�ķ�Ӧ����Ϊ
��8��д��G��H�ķ�Ӧ����ʽ��
 ��
��
| ||
| ����(����) |

 +RCl��
+RCl�� +NaCl
+NaClI������ƽF�ǽ�Ѫ֬�������̴���ҩ�����һ���ϳ�·�����£�

��1��AΪһԪ���ᣬ8.8gA������NaHCO3��Һ��Ӧ����2.24L CO2����״������A�ķ���ʽΪ
C4H8O2
C4H8O2
����2��д������A����ʽ�����м������Ľṹ��ʽ��


��3��B���ȴ����ᣬ��˴Ź��������������壬д��B��C�ķ�Ӧ����ʽ��


��4��C+E��F�ķ�Ӧ����Ϊ
ȡ����Ӧ
ȡ����Ӧ
����5��д��A��F�Ľṹ��ʽ��A��




��6��D�ı������������⣬�����������ŵ�����Ϊ
�ǻ�����ԭ��
�ǻ�����ԭ��
��д��a��b���������Լ���a��Cl2
Cl2
��b��NaOH
NaOH
��II��������·�ߣ���C�ɺϳɸ߾���H��
C
| ||
| �� |
| һ������ |
��7��C��G�ķ�Ӧ����Ϊ
��ȥ��Ӧ
��ȥ��Ӧ
����8��д��G��H�ķ�Ӧ����ʽ��


������������������������������ϻ�ѧ����ʽ�ɼ���A�ķ���ʽΪC4H8O2��B���ȴ����ᣬ�Һ˴Ź��������������壬���Ƴ�B�Ľṹ��ʽΪ ������ȷ��AΪ
������ȷ��AΪ ��CΪ
��CΪ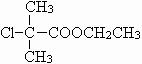 ��FΪ
��FΪ ����E�Ľṹ��ʽ��D�ı������������⣬����ȷ��DΪ
����E�Ľṹ��ʽ��D�ı������������⣬����ȷ��DΪ ������л���Ľṹ�жϾ��е����ʣ�
������л���Ľṹ�жϾ��е����ʣ�
 ������ȷ��AΪ
������ȷ��AΪ ��CΪ
��CΪ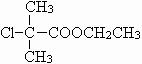 ��FΪ
��FΪ ����E�Ľṹ��ʽ��D�ı������������⣬����ȷ��DΪ
����E�Ľṹ��ʽ��D�ı������������⣬����ȷ��DΪ ������л���Ľṹ�жϾ��е����ʣ�
������л���Ľṹ�жϾ��е����ʣ�����⣺��1����A�ķ���ʽΪCnH2nO2�����У�
CnH2nO2+NaHCO3��CnH2n-1O2Na+CO2��+H2O
��14n+32��22.4L
8.8 2.24L
��
=
��
���n=4����A�ķ���ʽΪC4H8O2���ʴ�Ϊ��C4H8O2��
��2��A����ʽΪC4H8O2�����м������ĽṹΪ ��RΪ�����������֣���Ϊ-CH2CH2CH3��-CH��CH3��CH3������ͬ���칹����
��RΪ�����������֣���Ϊ-CH2CH2CH3��-CH��CH3��CH3������ͬ���칹���� ���֣�
���֣�
�ʴ�Ϊ�� ��
��
��3������B���ȴ����ᣬ�Һ˴Ź��������������壬���Ƴ�B�Ľṹ��ʽΪ ������ȷ��AΪ
������ȷ��AΪ ��CΪ
��CΪ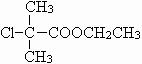 ��FΪ
��FΪ ��B��C����������Ӧ����Ӧ�Ļ�ѧ����ʽΪ
��B��C����������Ӧ����Ӧ�Ļ�ѧ����ʽΪ ��
��
�ʴ�Ϊ�� ��
��
��4��C+E��F�ķ�Ӧ�ɿ��� ȡ��-Cl�ķ�Ӧ���ʴ�Ϊ��ȡ����Ӧ��
ȡ��-Cl�ķ�Ӧ���ʴ�Ϊ��ȡ����Ӧ��
��5���������ƶϿ�֪AΪ ��FΪ
��FΪ ��
��
�ʴ�Ϊ�� ��
�� ��
��
��6����E�Ľṹ��ʽ��D�ı������������⣬����ȷ��DΪ �����к��еĹ��������ǻ�����ԭ�ӣ��DZ��Ӻ�Cl2��Ӧ�IJ����NaOH��Na2CO3��Һ��Ӧ����E��
�����к��еĹ��������ǻ�����ԭ�ӣ��DZ��Ӻ�Cl2��Ӧ�IJ����NaOH��Na2CO3��Һ��Ӧ����E��
�ʴ�Ϊ���ǻ�����ԭ�ӣ� Cl2��NaOH��Һ��
��7��C��NaOH�Ҵ���Һ�м��ȷ�����ȥ��Ӧ������ ��G�����ʴ�Ϊ����ȥ��Ӧ��
��G�����ʴ�Ϊ����ȥ��Ӧ��
��8��G�Ӿ�����H����Ӧ�Ļ�ѧ����ʽΪ ��
��
�ʴ�Ϊ�� ��
��
CnH2nO2+NaHCO3��CnH2n-1O2Na+CO2��+H2O
��14n+32��22.4L
8.8 2.24L
��
| 14n+32 |
| 8.8 |
| 22.4 |
| 2.24 |
���n=4����A�ķ���ʽΪC4H8O2���ʴ�Ϊ��C4H8O2��
��2��A����ʽΪC4H8O2�����м������ĽṹΪ
 ��RΪ�����������֣���Ϊ-CH2CH2CH3��-CH��CH3��CH3������ͬ���칹����
��RΪ�����������֣���Ϊ-CH2CH2CH3��-CH��CH3��CH3������ͬ���칹���� ���֣�
���֣��ʴ�Ϊ��
 ��
����3������B���ȴ����ᣬ�Һ˴Ź��������������壬���Ƴ�B�Ľṹ��ʽΪ
 ������ȷ��AΪ
������ȷ��AΪ ��CΪ
��CΪ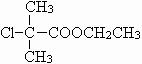 ��FΪ
��FΪ ��B��C����������Ӧ����Ӧ�Ļ�ѧ����ʽΪ
��B��C����������Ӧ����Ӧ�Ļ�ѧ����ʽΪ ��
���ʴ�Ϊ��
 ��
����4��C+E��F�ķ�Ӧ�ɿ���
 ȡ��-Cl�ķ�Ӧ���ʴ�Ϊ��ȡ����Ӧ��
ȡ��-Cl�ķ�Ӧ���ʴ�Ϊ��ȡ����Ӧ����5���������ƶϿ�֪AΪ
 ��FΪ
��FΪ ��
���ʴ�Ϊ��
 ��
�� ��
����6����E�Ľṹ��ʽ��D�ı������������⣬����ȷ��DΪ
 �����к��еĹ��������ǻ�����ԭ�ӣ��DZ��Ӻ�Cl2��Ӧ�IJ����NaOH��Na2CO3��Һ��Ӧ����E��
�����к��еĹ��������ǻ�����ԭ�ӣ��DZ��Ӻ�Cl2��Ӧ�IJ����NaOH��Na2CO3��Һ��Ӧ����E���ʴ�Ϊ���ǻ�����ԭ�ӣ� Cl2��NaOH��Һ��
��7��C��NaOH�Ҵ���Һ�м��ȷ�����ȥ��Ӧ������
 ��G�����ʴ�Ϊ����ȥ��Ӧ��
��G�����ʴ�Ϊ����ȥ��Ӧ����8��G�Ӿ�����H����Ӧ�Ļ�ѧ����ʽΪ
 ��
���ʴ�Ϊ��
 ��
�����������⿼���л���ĺϳɣ���Ŀ�ѶȽϴ���ע����������Ϣ���������Ƶķ����ƶϣ���ȷ�ƶ�A����ɺͽṹ�ǽ�����Ĺؼ���

��ϰ��ϵ�д�
�����Ŀ



 9Fe3++NO��+14H2O
9Fe3++NO��+14H2O


