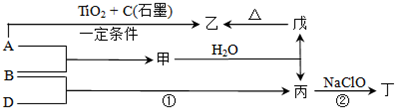
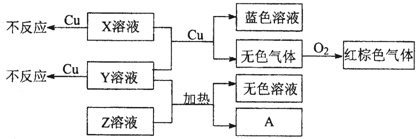
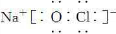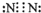��Ŀ����
�ס��ҡ�����������Ϊԭ��������������Ķ�����Ԫ�ء��ס�������ͬһ���壬���������촦��ͬһ���ڣ���ԭ�ӵ������������Ǽס��ҡ���ԭ������������֮�͡��ס�����ɵij�������X��ʹʪ��ĺ�ɫʯ����ֽ��������ĵ�����X��Ӧ�������ҵĵ��ʣ�ͬʱ������������ˮ�������ԵĻ�����Y��Z��0.1 mol/L��Y��ҺpH��1�����ĵ��ʼ������Ԫ������������ˮ�������Һ��Ӧ������L��Ҳ����Z��ˮ��Һ��Ӧ�����Σ����������ɻ�����M����ش��������⣺
(1) �����ӵĽṹʾ��ͼΪ��_________________��
(2) д���ҵĵ��ʵĵ���ʽ��_________________��
(3) ��ĵ�����X��Ӧ���ɵ�Y��Z�����ʵ���֮��Ϊ2��4����Ӧ�б������������뱻��ԭ�����ʵ����ʵ���֮��Ϊ__________��
(4) д������Z��ϡ��Һ�������L��ϡ��Һ�з�����Ӧ�����ӷ���ʽ��___________________________________��
(1) �����ӵĽṹʾ��ͼΪ��_________________��
(2) д���ҵĵ��ʵĵ���ʽ��_________________��
(3) ��ĵ�����X��Ӧ���ɵ�Y��Z�����ʵ���֮��Ϊ2��4����Ӧ�б������������뱻��ԭ�����ʵ����ʵ���֮��Ϊ__________��
(4) д������Z��ϡ��Һ�������L��ϡ��Һ�з�����Ӧ�����ӷ���ʽ��___________________________________��
(1)
(2)
(3)2��3
(4)H++AlO2-+H2O �� Al(OH)3��

(2)

(3)2��3
(4)H++AlO2-+H2O �� Al(OH)3��

��ϰ��ϵ�д�
 ���Ž�������С״Ԫϵ�д�
���Ž�������С״Ԫϵ�д�
�����Ŀ
�ס��ҡ�����������Ϊ������ԭ�����������������������Ԫ�أ��ҡ���ͬ���壬�����ҵ�ԭ������֮�͵������ԭ�����������Ƕ���������Ԫ����ԭ�Ӱ뾶����Ԫ�أ���Ԫ���ڵؿ��к����ӽ���Ԫ�صĵ�һλ������˵����ȷ���ǣ�������
| A�������Ӱ뾶�����������ң��� | B����̬�⻯����ȶ��ԣ��ף��� | C��������γɵĻ��������һ�� | D�����������������������Ӧ��ˮ����֮���������ܷ�Ӧ |