��Ŀ����
�ס��ҡ�����������Ϊ���ֶ�����Ԫ�أ���ԭ���������������붡��������ֱ�ͬ���壬���ԭ�������������ȴ������2���������ҿ�����ԭ�Ӹ�����3��1�γɻ�����A����ÿ��A�����к���10�����ӣ���ش�
��1�����ԭ�ӽṹʾ��ͼ��
 ���ҵ��ʷ��ӵĵ���ʽ��
���ҵ��ʷ��ӵĵ���ʽ��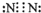
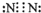 ��
��
��2��A����ˮ������Һ�ʼ��Ե�ԭ���ǣ��õ��뷽��ʽ��ʾ��
��3�����붡���γ�һ�����ӻ�����û�������H2O��Ӧ�õ�ǿ����Һ��H2����÷�Ӧ�У��������뻹ԭ�������ʵ���֮����
��4��X��Y��ZΪ����ǿ����ʣ��ֱ�����������Ԫ���е�������ɣ�X��Y��Z��ϡ��Һ֮���������ת����ϵ��
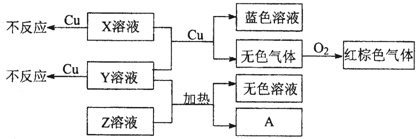
��Y��Z��Ϻ���ȵõ�A���ӷ���ʽ��
�ڽ���Cu��X��Y�Ļ����Һ��Ӧ�����ӷ���ʽ��
��1�����ԭ�ӽṹʾ��ͼ��


��2��A����ˮ������Һ�ʼ��Ե�ԭ���ǣ��õ��뷽��ʽ��ʾ��
NH3?H2O?NH4++OH-
NH3?H2O?NH4++OH-
����3�����붡���γ�һ�����ӻ�����û�������H2O��Ӧ�õ�ǿ����Һ��H2����÷�Ӧ�У��������뻹ԭ�������ʵ���֮����
1��1
1��1
����4��X��Y��ZΪ����ǿ����ʣ��ֱ�����������Ԫ���е�������ɣ�X��Y��Z��ϡ��Һ֮���������ת����ϵ��

��Y��Z��Ϻ���ȵõ�A���ӷ���ʽ��
NH4++OH-
NH3��+H2O
| ||
NH4++OH-
NH3��+H2O
��
| ||
�ڽ���Cu��X��Y�Ļ����Һ��Ӧ�����ӷ���ʽ��
3Cu+8H++2NO3-=3Cu2++2NO��+4H2O
3Cu+8H++2NO3-=3Cu2++2NO��+4H2O
�����������ԭ�������������ȴ������2����ӦΪSԪ�أ������죬���ΪOԪ�أ������ҿ�����ԭ�Ӹ�����3��1�γɻ�����A����ÿ��A�����к���10�����ӣ�ӦΪNH3�����ΪHԪ�أ���ΪNԪ�أ����붡������ԭ��������ϵ��֪��ΪNaԪ�أ���϶�ӦԪ�ػ�����������Լ���ĿҪ��ɽ����⣮
����⣺���ԭ�������������ȴ������2����ӦΪSԪ�أ������죬���ΪOԪ�أ������ҿ�����ԭ�Ӹ�����3��1�γɻ�����A����ÿ��A�����к���10�����ӣ�ӦΪNH3�����ΪHԪ�أ���ΪNԪ�أ����붡������ԭ��������ϵ��֪��ΪNaԪ�أ�
��1�������Ϸ��ӿ�֪��ΪS��ԭ�Ӻ�����3�����Ӳ㣬����������Ϊ3����ԭ�ӽṹʾ��ͼΪ ���ҵ���ΪN2������ʽΪ
���ҵ���ΪN2������ʽΪ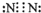 ��
��
�ʴ�Ϊ�� ��
��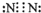 ��
��
��2������ˮ��Һ�д���NH3?H2O��Ϊ������ʣ��ɵ����OH-����Һ�ʼ��ԣ����ӷ���ʽΪNH3?H2O?NH4++OH-��
�ʴ�Ϊ��NH3?H2O?NH4++OH-��
��3�����붡���γ�һ�����ӻ�����ΪNaH����ˮ��Ӧ�ķ���ʽΪNaH+H2O=NaOH+H2�����÷�Ӧ�У���������H2O�뻹ԭ����NaH���ɷ���ʽ��֪�������ʵ���֮����1��1���ʴ�Ϊ��1��1��
��4��Y��Һ��Z��Һ��������NH3����Y��Һ��Z��ҺΪ�����X��Һ��Y��Һ������ͭ����Ӧ�����������ͭ��Ӧ������ɫ���壬��������������Ӧ�ʺ���ɫ�����Ժ���ɫ����ΪNO2����ɫ����ΪNO������X��Һ��Y��ҺΪ���������ᣬ����Y��Һ��Z��ҺΪ���������YΪ����泥�ZΪ�������ƣ�XΪ���ᣮ
��YΪ�������Һ��ZΪNaOH��Һ�������ڼ��������¿ɷ�Ӧ������
��Ӧ�����ӷ���ʽΪNH4++OH-
NH3��+H2O��
�ʴ�Ϊ��NH4++OH-
NH3��+H2O��
��Cu��X��Y�Ļ����Һ��Ӧ�����ӷ���ʽ��3Cu+8H++2NO3-=3Cu2++2NO��+4H2O��
�ʴ�Ϊ��3Cu+8H++2NO3-=3Cu2++2NO��+4H2O��
��1�������Ϸ��ӿ�֪��ΪS��ԭ�Ӻ�����3�����Ӳ㣬����������Ϊ3����ԭ�ӽṹʾ��ͼΪ
 ���ҵ���ΪN2������ʽΪ
���ҵ���ΪN2������ʽΪ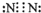 ��
���ʴ�Ϊ��
 ��
��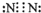 ��
����2������ˮ��Һ�д���NH3?H2O��Ϊ������ʣ��ɵ����OH-����Һ�ʼ��ԣ����ӷ���ʽΪNH3?H2O?NH4++OH-��
�ʴ�Ϊ��NH3?H2O?NH4++OH-��
��3�����붡���γ�һ�����ӻ�����ΪNaH����ˮ��Ӧ�ķ���ʽΪNaH+H2O=NaOH+H2�����÷�Ӧ�У���������H2O�뻹ԭ����NaH���ɷ���ʽ��֪�������ʵ���֮����1��1���ʴ�Ϊ��1��1��
��4��Y��Һ��Z��Һ��������NH3����Y��Һ��Z��ҺΪ�����X��Һ��Y��Һ������ͭ����Ӧ�����������ͭ��Ӧ������ɫ���壬��������������Ӧ�ʺ���ɫ�����Ժ���ɫ����ΪNO2����ɫ����ΪNO������X��Һ��Y��ҺΪ���������ᣬ����Y��Һ��Z��ҺΪ���������YΪ����泥�ZΪ�������ƣ�XΪ���ᣮ
��YΪ�������Һ��ZΪNaOH��Һ�������ڼ��������¿ɷ�Ӧ������
��Ӧ�����ӷ���ʽΪNH4++OH-
| ||
�ʴ�Ϊ��NH4++OH-
| ||
��Cu��X��Y�Ļ����Һ��Ӧ�����ӷ���ʽ��3Cu+8H++2NO3-=3Cu2++2NO��+4H2O��
�ʴ�Ϊ��3Cu+8H++2NO3-=3Cu2++2NO��+4H2O��
���������⿼��������ƶϣ�������Ԫ�����ڱ�λ����ԭ�ӽṹ��ϵ�Ŀ��飬��Ŀ�Ѷ��еȣ�ע����ȷ�ƶ�Ԫ�ص�����Ϊ�����Ĺؼ���

��ϰ��ϵ�д�
 ��Ӣ���㿨ϵ�д�
��Ӣ���㿨ϵ�д� Ӧ����㲦ϵ�д�
Ӧ����㲦ϵ�д�
�����Ŀ
�ס��ҡ�����������Ϊ������ԭ�����������������������Ԫ�أ��ҡ���ͬ���壬�����ҵ�ԭ������֮�͵������ԭ�����������Ƕ���������Ԫ����ԭ�Ӱ뾶����Ԫ�أ���Ԫ���ڵؿ��к����ӽ���Ԫ�صĵ�һλ������˵����ȷ���ǣ�������
| A�������Ӱ뾶�����������ң��� | B����̬�⻯����ȶ��ԣ��ף��� | C��������γɵĻ��������һ�� | D�����������������������Ӧ��ˮ����֮���������ܷ�Ӧ |