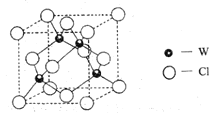
��Ŀ����
����Ŀ������������һ�ְ�ɫ�й���Ƭ״�ᾧ���ɫ�ᾧ��ĩ���ǻǰ���ҩ���ԭ �ϣ�������ֹʹ�������ȼ�����������Ⱦ���м��塣�����������Ʊ�ԭ��Ϊ��
![]() +CH3COOH
+CH3COOH![]() +H2O
+H2O

ע�����η������������൱�ڶ����������ڷе���̫��Ļ����ķ��롣

ʵ�鲽��:
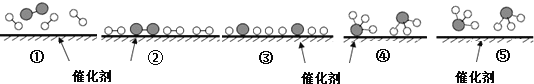
���� 1����Բ����ƿ�м�����ˮ���� 9.2 mL�������� 17.4 mL��п��0.1 g����װ�����������ʯ�����ڼ����¶ȣ�ʹ���������¶ȿ�����105�� ���ң���ӦԼ 60��80 min����Ӧ���ɵ�ˮ���������ᱻ������ ���� 2���ڽ����£����Ƚ���ƿ�е�������ϸ��״����ʢ�� 100 mL �� ˮ���ձ��У����ҽ��裬����ȴ���ᾧ�����ˡ�ϴ�ӡ�����õ����� ������Ʒ������ 3�����˴��������������ؽᾧ�����ɣ����أ�������ʡ�
(1)���� 1 ����ѡԲ����ƿ����ѹ����_________(�����)��
a��25 mL b��50 mL c��150 mL d��200 mL
(2)ʵ���м�������п�۵�Ŀ����___________________________________________________________________________��
(3)�ӻ�ѧƽ��ĽǶȷ��������Ʒ������϶˵��¶��� 105�����ҵ�ԭ��____________________________________________________________________________��
(4)ϴ������������Ʒ����ʵķ�����_____(�����)��
a����������ˮϴ b����������ˮϴ c���þƾ�ϴ
(5)����������Ʒ�����ʶ���ɫ�������ؽᾧ�����ᴿ���������£���ˮ�ܽ⡢
_______________�����ˡ�ϴ�ӡ�����(ѡ����ȷ�IJ���������)��
a�������ᾧ b����ȴ�ᾧ c�����ȹ��� d���������̿
(6)��ʵ�����յõ���Ʒ 8.1g�������������IJ�����______________ ����
(7)��ͼ��װ���� 1 ��������ָ������֮��____________________________________________________________��
���𰸡�b ��ֹ�����ڷ�Ӧ�����б����� ���Ϸ������Ӧ���������ɵ�ˮ���ٽ���Ӧ������У���߲�Ʒ�IJ��� a d c b 60 β�ӹܺ���ƿ�ܷ�
��������
(1)Բ����ƿ����ʢ��Һ�岻�ܳ������ݻ���2/3��Ҳ��������1/3��
(2)���ݱ������л�ԭ�Է�����
(3) ˮ�ķе���100�棬������105�����ң��Ϳ��Բ��Ϸֳ���Ӧ���������ɵ�ˮ��
(4)������������������ˮ��������ˮ�������Ҵ������ѷ�����
(5)������������ˮ�ܽ⣬�û���̿����ɫ�أ����ȹ��ˣ������ȴ�ᾧ���õ�����������
(6)����=ʵ�ʲ��������۲�����100%��
(7)ͼ��װ����β�ӹ�����ƿ�ӿڲ����ܷ⡣
(1)Բ����ƿ����ʢ��Һ�岻�ܳ������ݻ���2/3��Ҳ��������1/3����ƿ�м�����ˮ���� 9.2 mL�������� 17.4 mL��п��0.1 g������Բ����ƿ����ѹ����50 mL��ѡb��
(2)�������л�ԭ�ԣ����ױ������е�����������Ϊ��ֹ�����ڷ�Ӧ�����б����������뻹ԭ��Zn�ۡ�
(3)ˮ�ķе���100�棬������105�����ң��Ϳ��Բ��Ϸֳ���Ӧ���������ɵ�ˮ���ٽ���Ӧ������У����������IJ��ʡ�
(4)��������������ˮ��������ˮ�������Ҵ������ѣ�Ϊ��������������ʧ��ϴ������������Ʒ����ʵķ�������������ˮϴ��ѡa��
(5)������������������ˮ��������ˮ������������Ʒ�����ʶ���ɫ�����ؽᾧ�����ᴿ�IJ���Ϊ��������������ˮ�ܽ⣬�û���̿����ɫ�أ����ȹ��ˣ������ȴ�ᾧ���õ�����������������ȷ�IJ�����d c b��
��6��n(����)= (9.1mL ��1.02g/mL)��93g/mol=0.1mol��n(����)=( 17.4mL ��1.05g/mL)��60g/mol=0.305mol�����ڱ��������ʵ������������ɵ��������������ʵ���Ҫ�Բ������ı������㣬���۲���Ϊ0.1mol����ʵ�ʲ���n(��������)= 8.1g��135g/mol=0.06mol���������������IJ���Ϊ0.06mol��0.1mol��100%=60%��
(7)ͼ��װ����β�ӹ�����ƿ�ӿڲ����ܷ⣬����֮����β�ӹܺ���ƿ�ܷ⡣