��Ŀ����
I.���������ʽ��з��ࣺ
��![]() H��
H��![]() H �� ��O2��O3 ���� CH2=CHCH3 ��CH2=C(CH3)2
H �� ��O2��O3 ���� CH2=CHCH3 ��CH2=C(CH3)2
���Ҵ���C2H5OH������ѣ�CH3��O��CH3�� ��
�������飨CH3CH2 CH2 CH3�����춡�飨 �� ��
�� ��
���飨CH4������飨C3H8������C6H5OH ��C6H5CH2OH(C6H5-Ϊ����)
��1����Ϊͬλ�ص��� ������š���ͬ����
��2����Ϊͬϵ����� ��
��3����Ϊͬ���칹����� ��
(4) ��Ϊͬ����������� .
II.���ڢٱ� �ڱ�ϩ �ۼױ� �ܱ��� �ݱ����У�������š���ͬ��
��1���ܺͽ����Ʒ�Ӧ�ų�H2������
��2������NaOH��Һ��Ӧ����
��3������������Ũ��ˮ��Ӧ����
��4������ԭ�� һ������ͬһƽ�����
��5����ʹ����KMnO4��Һ��ɫ����
(1).��
(2).�ۢ�
(3).�ݢ�
(4).��
II(1).�ܢ�
(2).��
(3).�ڢ�
(4).��
(5).�ڢۢ�ÿ��2��,�д���������ѡ��1��

��ϰ��ϵ�д�
�����Ŀ

 �������������������������������ռ�����
�������������������������������ռ����� =3Cu��NO3��2+2NO��+4H2O
=3Cu��NO3��2+2NO��+4H2O

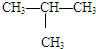 ����
����