��Ŀ����
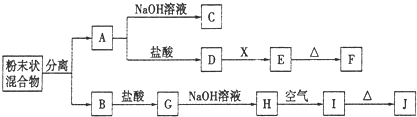
ij��ѧ��ȤС���ú�A��B���ֽ������ʵķ�ĩ״������������ʵ�飬��ת����ϵ����ͼ��ʾ�����ַ�Ӧ���������δ�г���������EΪ��ɫ������IΪ���ɫ����������ת����ϵ�����õ��Լ����������ģ�

��1��д���������ʵĻ�ѧʽ��F
��2��������������ֽ������뿪����ķ�����
��3��D��E��ת���У����������X������
A������NaCl��Һ B��NaOH��Һ C����ˮ D��Ba��OH��2��Һ
��4��д������ת���Ļ�ѧ����ʽ��
A��C��
H��I��

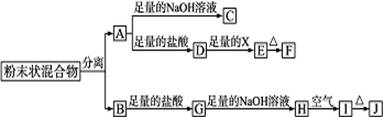
��1��д���������ʵĻ�ѧʽ��F
Al2O3
Al2O3
��GFeCl2
FeCl2
����2��������������ֽ������뿪����ķ�����
�ô���������ĩ״���������������ڴ�������
�ô���������ĩ״���������������ڴ�������
����3��D��E��ת���У����������X������
C
C
��A������NaCl��Һ B��NaOH��Һ C����ˮ D��Ba��OH��2��Һ
��4��д������ת���Ļ�ѧ����ʽ��
A��C��
2Al+2NaOH+2H2O=2NaAlO2+3H2��
2Al+2NaOH+2H2O=2NaAlO2+3H2��
��H��I��
4Fe��OH��2+O2+2H2O=4Fe��OH��3
4Fe��OH��2+O2+2H2O=4Fe��OH��3
��������IΪ���ɫ�����ж�ΪFe��OH��3�����ȷ�Ӧ����JΪFe2O3��H�ڿ���������Ϊ���������ƶ�HΪFe��OH��2��b+HCl=G���ƶ�GΪFeCl2��BΪFe����A��B���ֽ������ʵķ�ĩ״�������BΪFe��A�ܺ��������Ʒ�Ӧ�к����ᷴӦ˵��Ϊ����Ԫ���ж�ΪAl������CΪNaAlO2��DΪAlCl3������EΪ��ɫ����Al��OH��3����XΪ�Ʊ����������ķ�Ӧ���ѡ��ˮ����XΪNH3?H2O��FΪAl2O3�������ƶ����ʷ����ش����⣮
����⣺��1�������ж�FG�Ļ�ѧʽΪ��FΪAl2O3��GΪFeCl2���ʴ�Ϊ��Al2O3��FeCl2��
��2������������ֽ������뿪����ķ��������������Ա���������������Ϊ���ô���������ĩ״���������������ڴ������棬
�ʴ�Ϊ���ô���������ĩ״���������������ڴ������棻
��3��D��E��ת������X���Ȼ�����Ӧ�������������ķ�Ӧ������ʵ������ȡ���������ķ������������������ʷ������������������ڹ���ǿ����Һ�У����Լ��������X�����ǰ�ˮ��
�ʴ�Ϊ��C��
��4��A��C��Al���������Ʒ�Ӧ����ƫ�����ƺ���������Ӧ�Ļ�ѧ����ʽΪ2Al+2NaOH+2H2O=2NaAlO2+3H2����H��I������������������Ϊ�����������÷�Ӧ�Ļ�ѧ����ʽΪ��4Fe��OH��2+O2+2H2O=4Fe��OH��3���ʴ�Ϊ��2Al+2NaOH+2H2O=2NaAlO2+3H2����4Fe��OH��2+O2+2H2O=4Fe��OH��3��
��2������������ֽ������뿪����ķ��������������Ա���������������Ϊ���ô���������ĩ״���������������ڴ������棬
�ʴ�Ϊ���ô���������ĩ״���������������ڴ������棻
��3��D��E��ת������X���Ȼ�����Ӧ�������������ķ�Ӧ������ʵ������ȡ���������ķ������������������ʷ������������������ڹ���ǿ����Һ�У����Լ��������X�����ǰ�ˮ��
�ʴ�Ϊ��C��
��4��A��C��Al���������Ʒ�Ӧ����ƫ�����ƺ���������Ӧ�Ļ�ѧ����ʽΪ2Al+2NaOH+2H2O=2NaAlO2+3H2����H��I������������������Ϊ�����������÷�Ӧ�Ļ�ѧ����ʽΪ��4Fe��OH��2+O2+2H2O=4Fe��OH��3���ʴ�Ϊ��2Al+2NaOH+2H2O=2NaAlO2+3H2����4Fe��OH��2+O2+2H2O=4Fe��OH��3��
���������⿼��������ת����ϵ�ķ����жϣ��������ʵ�Ӧ�ã���Ϥ�����仯��������仯�������ʵ�Ӧ���ǽ��Ĺؼ�����Ŀ�Ѷ��еȣ�

��ϰ��ϵ�д�
 ��ǰ�κ�ͬ����ϰϵ�д�
��ǰ�κ�ͬ����ϰϵ�д� ����С��ҵϵ�д�
����С��ҵϵ�д� �Ƹ�С״Ԫ����������ϰ��ϵ�д�
�Ƹ�С״Ԫ����������ϰ��ϵ�д� �ɹ�ѵ���ƻ�ϵ�д�
�ɹ�ѵ���ƻ�ϵ�д� ����ѵ����ֱͨ�п�����ϵ�д�
����ѵ����ֱͨ�п�����ϵ�д� һ���㶨ϵ�д�
һ���㶨ϵ�д�
�����Ŀ