��Ŀ����
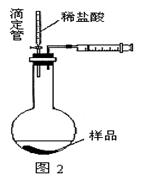
��12�֣�ijѧ��ʵ����ȤС������ͼ1װ������ɡ�NaHCO3��NaCl�������NaHCO3�����IJⶨ����ʵ�顣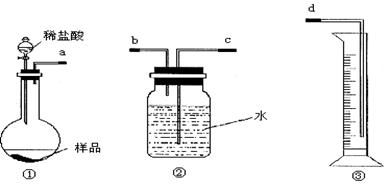
ͼ1
��1���������ӿ�����˳����_________________���ýӿ���ĸ��д����
��2����װ�����Ӻö�δװҩƷǰ�����������ԵIJ�����_________________________��
��3����ͬѧ��Ϊ�����������ϴ�������¸Ľ���ʩ������Ϊ���е���______��������ţ�
| A���ڵ�������֮ǰ��Ӧ�ž�װ���ڵ�CO2���� |
| B����װ�â���ϡ���ỻ��ϡ���ᣬװ�â���ˮ���ɱ���NaHCO3��Һ |
| C����װ�â���ˮ���ɱ���Na2CO3��Һ |
| D���μ�����˹��� |
���ú�V1��V2��V3��m��ʽ�ӱ�ʾ����

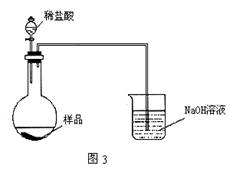
��5����ͬѧ����ͼ3װ�ã�ͨ���ⶨ�ձ�������������Ϊ�ҡ�����ͬѧ�ķ����У�˭�ķ���������__________��������:_____________��
��1��a��b��c��d��2�֣�
��2����1���ڢ��м�ˮ��û�����ܣ��رշ�Һ©��������������סԲ����ƿ����ƾ����ȣ��������г�������Һ����������˵�������Ժã���2���ڢ��м�ˮ��û���ܣ��رշ�Һ©���������þƾ����ȣ������е��ܿڲ������ȵ����ݣ�ֹͣ���Ⱥ���ĩ���γ�һ��ˮ���ұ���һ��ʱ�䲻�½�����˵�������Ժá���2�֣�
��3��BD��2�֣�
��4��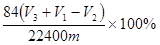 ��2�֣�
��2�֣�
��5����ͬѧ��1�֣���ͬѧ�ķ����У����ɵ�CO2û��ȫ��NaOH��Һ���ա���2�֣�
����

��ϰ��ϵ�д�
�����Ŀ
������ָpH��5.6���ꡢѩ�ȸ�����ʽ�Ĵ�����ˮ�������������γ��������Ҫ����֮һ������Ҫ���ɺ���ȼ�ϣ�ú��ʯ�ͣ�ȼ�պͽ���ұ�����ͷŵĶ�������������ɣ������Σ�������ٶ����������Ⱦ����ŷţ����������Ƿdz���Ҫ�ģ�ijУ��ѧ��ȤС���ѧ�����ֳ���������꼰�����ж�������ĺ��������˲ⶨ��
��һ�飬ȡ�ս������������ˮ��������������ʵ�飺
��1����һƬ��ɫ��õ�廨�����һ��ˮ����
��2��ÿ��һ��ʱ��ⶨ��pH�������������ʾ��
��3������ˮ�����еμ��Ȼ�����Һ���а�ɫ���dz���
����ͬʱ�������pH
��1��һ��ʱ��۲쵽õ�廨��ɫ��dz�����ܵ�ԭ����______��
��2�����ɰ�ɫ���ǵĻ�ѧ����ʽ______��
��3����������pH���ݱ仯������Ϊ���ܵ�ԭ���ǣ��û�ѧ����ʽ��ʾ����______��
�ڶ��飬����������װ�ö�������������SO2�ĺ�����
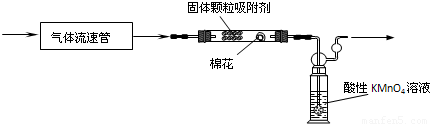
��4������ͬѧ�����ϵõ��ķ�Ӧԭ��Ϊ��SO2������KMnO4��Һ��Ӧ�Ļ�ѧ����ʽ��5SO2+2KMnO4+2H2O=2H2SO4+2MnSO4+K2SO4��
�÷�Ӧ��������______
��5��KMnO4��Һ�е���ĩ��������״��ṹ��������______��
��6����ʵ�����Ѿ�֪������������������a L/min������KMnO4��Һ�����b L����Ũ��Ϊc mol/L����������ͨ�뵽��ɫǡ����ȥ����ʱ5���ӣ���˴�ȡ�����Ŀ����ж���������Ϊ______g/L��
��һ�飬ȡ�ս������������ˮ��������������ʵ�飺
��1����һƬ��ɫ��õ�廨�����һ��ˮ����
��2��ÿ��һ��ʱ��ⶨ��pH�������������ʾ��
��3������ˮ�����еμ��Ȼ�����Һ���а�ɫ���dz���
����ͬʱ�������pH
| �ⶨʱ��/Сʱ�� | 1 | 2 | 4 | |
| pH | 4.73 | 4.62 | 4.56 | 4.55 |
��2�����ɰ�ɫ���ǵĻ�ѧ����ʽ______��
��3����������pH���ݱ仯������Ϊ���ܵ�ԭ���ǣ��û�ѧ����ʽ��ʾ����______��
�ڶ��飬����������װ�ö�������������SO2�ĺ�����

��4������ͬѧ�����ϵõ��ķ�Ӧԭ��Ϊ��SO2������KMnO4��Һ��Ӧ�Ļ�ѧ����ʽ��5SO2+2KMnO4+2H2O=2H2SO4+2MnSO4+K2SO4��
�÷�Ӧ��������______
��5��KMnO4��Һ�е���ĩ��������״��ṹ��������______��
��6����ʵ�����Ѿ�֪������������������a L/min������KMnO4��Һ�����b L����Ũ��Ϊc mol/L����������ͨ�뵽��ɫǡ����ȥ����ʱ5���ӣ���˴�ȡ�����Ŀ����ж���������Ϊ______g/L��