��Ŀ����
���������Ǵ�����Ⱦ��֮һ��������������ķ����ж��֡�
��1�����ü������ԭ���������֪��
��CH4 (g)��4NO2(g)��4NO(g)��CO2(g)��2H2O(g��?? ��H ����574 kJ/mol
��CH4(g)��4NO(g���� 2N2(g)��CO2(g)��2H2O(g��? ��H ����1160 kJ/mol
��CH4 ��NO2 ��ԭΪN2 ���Ȼ�ѧ����ʽΪ��??????????? ???????????? ��
 ��2������NH3����ԭ����������SCR����)���ü�����ĿǰӦ����㷺���������������ѳ������� ��Ӧ�Ļ�ѧ����ʽΪ:
��2������NH3����ԭ����������SCR����)���ü�����ĿǰӦ����㷺���������������ѳ������� ��Ӧ�Ļ�ѧ����ʽΪ: Ϊ��ߵ��������ת���ʿɲ�ȡ�Ĵ�ʩ��???????? ????????????????????????? ��д��1�����ɣ���
Ϊ��ߵ��������ת���ʿɲ�ȡ�Ĵ�ʩ��???????? ????????????????????????? ��д��1�����ɣ���
��3������ClO2 ����������������ת����������:? NO NO2
NO2 N2����֪��Ӧ���Ļ�ѧ����ʽΪ2NO+ ClO2 + H2O ��NO2 + HNO3 + HCl������Ӧ���Ļ�ѧ����ʽ��???????? ????? ��������11.2 L N2����״������������ClO2 ??????? g ��
N2����֪��Ӧ���Ļ�ѧ����ʽΪ2NO+ ClO2 + H2O ��NO2 + HNO3 + HCl������Ӧ���Ļ�ѧ����ʽ��???????? ????? ��������11.2 L N2����״������������ClO2 ??????? g ��
��4���û���̿��ԭ��������������йط�ӦΪ��C��s��+2NO��g�� N2 ��g��+CO2 ��g����H��ij�о�С����ij�ܱ���������һ�����Ļ���̿��NO�����£�T1���������·�Ӧ����Ӧ���е���ͬʱ���ø����ʵ�Ũ�����£�
N2 ��g��+CO2 ��g����H��ij�о�С����ij�ܱ���������һ�����Ļ���̿��NO�����£�T1���������·�Ӧ����Ӧ���е���ͬʱ���ø����ʵ�Ũ�����£�
Ũ��/mol?L-1/ ʱ��/min | NO | N2 | CO2 |
0 | 0.100 | 0 | 0 |
10 | 0.058 | 0.021 | 0.021 |
20 | 0.040 | 0.030 | 0.030 |
30 | 0.040 | 0.030 | 0.030 |
40 | 0.032 | 0.034 | 0.017 |
50 | 0.032 | 0.034 | 0.017 |
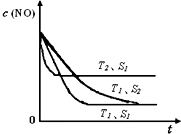
��T1��ʱ���÷�Ӧ��ƽ�ⳣ��K= ????????? ��������λС��������30min�ı�ijһ��������Ӧ���´ﵽƽ�⣬��ı������������ ????????????? ������30min�������¶���T2�����ﵽƽ��ʱ��������NO��N2��CO2��Ũ��֮��Ϊ5��3��3����÷�Ӧ����H ?? 0��������������=��������������
��1��CH4 (g)��2NO2(g)��N2(g)��CO2(g)��2H2O(g��?? ��H ����867 kJ/mol
��2������NH3��Ũ�Ȼ��С��Ӧ��ϵ��ѹǿ�ͷ�Ӧ��ϵ���¶ȵ�
��3��2NO2 + 4 Na2SO3=N2 + 4 Na2SO4??? 67.5? ��4����0.56? �� ����CO2Ũ��? �� ��
��������
�����������1���������ڣ���2�ɵ�CH4 (g)��2NO2(g)��N2(g)��CO2(g)��2H2O(g��?? ��H ����867 kJ/mol����2�����ڷ�Ӧ ������Ӧ�����������ķ��ȷ�Ӧ��������ߵ��������ת���ʿɲ�ȡ�Ĵ�ʩ������ͨ������NH3��Ũ�Ȼ��С��Ӧ��ϵ��ѹǿ�ͷ�Ӧ��ϵ���¶ȵȴ�ʩ��ʵʩ����3������Ŀ�ṩ����Ϣ��֪NO2��Na2SO3����������ԭ��Ӧ��2NO2 + 4 Na2SO3 =N2 + 4 Na2SO4���Ӷ�������NOx�Ի�����ɵ���Ⱦ���ɷ���ʽ�ù�ϵʽ��2ClO2��2NO2 ��N2��n(N2)=0.5mol,����n(ClO2)=1mol��m(ClO2)= 1mol��67.5g/mol=67.5g����4����T1��ʱ���÷�Ӧ��ƽ�ⳣ��
������Ӧ�����������ķ��ȷ�Ӧ��������ߵ��������ת���ʿɲ�ȡ�Ĵ�ʩ������ͨ������NH3��Ũ�Ȼ��С��Ӧ��ϵ��ѹǿ�ͷ�Ӧ��ϵ���¶ȵȴ�ʩ��ʵʩ����3������Ŀ�ṩ����Ϣ��֪NO2��Na2SO3����������ԭ��Ӧ��2NO2 + 4 Na2SO3 =N2 + 4 Na2SO4���Ӷ�������NOx�Ի�����ɵ���Ⱦ���ɷ���ʽ�ù�ϵʽ��2ClO2��2NO2 ��N2��n(N2)=0.5mol,����n(ClO2)=1mol��m(ClO2)= 1mol��67.5g/mol=67.5g����4����T1��ʱ���÷�Ӧ��ƽ�ⳣ�� .���ɱ������ݿ��Կ���30min��c(CO2)��С��c(N2)ȴ������c(NO)�������١�˵���ı����������Сc(CO2)������30min�������¶���T2�����ﵽƽ��ʱ��������NO��N2��CO2��Ũ��֮��Ϊ5��3��3��
.���ɱ������ݿ��Կ���30min��c(CO2)��С��c(N2)ȴ������c(NO)�������١�˵���ı����������Сc(CO2)������30min�������¶���T2�����ﵽƽ��ʱ��������NO��N2��CO2��Ũ��֮��Ϊ5��3��3�� <0.56��˵�������¶ȣ���ѧƽ�����淴Ӧ�����ƶ�������ƽ���ƶ�ԭ��:�����¶ȣ���ѧƽ�������ȷ�Ӧ�����ƶ����淴Ӧ����Ϊ���ȷ�Ӧ�����Ը÷�Ӧ������ӦΪ���ȷ�Ӧ����÷�Ӧ����H ��0��
<0.56��˵�������¶ȣ���ѧƽ�����淴Ӧ�����ƶ�������ƽ���ƶ�ԭ��:�����¶ȣ���ѧƽ�������ȷ�Ӧ�����ƶ����淴Ӧ����Ϊ���ȷ�Ӧ�����Ը÷�Ӧ������ӦΪ���ȷ�Ӧ����÷�Ӧ����H ��0��
���㣺���黯ѧ����ʽ���Ȼ�ѧ����ʽ����д����ѧƽ�ⳣ���ļ��㡢��������Ի�ѧƽ���ƶ���Ӱ�졣

 ���������Ǵ�����Ⱦ��֮һ��������������ķ����ж��֣�
���������Ǵ�����Ⱦ��֮һ��������������ķ����ж��֣�



