题目内容
已知热化学方程式:
H2O(g)=H2(g)+
O2(g);△H=+241.8kJ/mol
H2(g)+
O2(g)=H2O(l);△H=-285.8kJ/mol
当1g液态水变为水蒸气时,其热量变化是( )
H2O(g)=H2(g)+
| 1 |
| 2 |
H2(g)+
| 1 |
| 2 |
当1g液态水变为水蒸气时,其热量变化是( )
| A.吸热88kJ | B.吸热44kJ |
| C.放热44kJ | D.吸热2.44kJ |
H2O(g)=H2(g)+
O2(g);△H=+241.8kJ/mol①
H2(g)+
O2(g)=H2O(l);△H=-285.8kJ/mol②
将方程式①+②得H2O(g)=H2O(l);△H=+241.8kJ/mol-285.8kJ/mol=-44kJ/mol,
所以H2O(l)=H2O(g);△H=+44kJ/mol,
1g水的物质的量=
=
mol,当1g液态水变为水蒸气时,吸收热量=
mol×44kJ/mol=2.44kJ,
故选D.
| 1 |
| 2 |
H2(g)+
| 1 |
| 2 |
将方程式①+②得H2O(g)=H2O(l);△H=+241.8kJ/mol-285.8kJ/mol=-44kJ/mol,
所以H2O(l)=H2O(g);△H=+44kJ/mol,
1g水的物质的量=
| 1g |
| 18g/mol |
| 1 |
| 18 |
| 1 |
| 18 |
故选D.

练习册系列答案
 暑假作业海燕出版社系列答案
暑假作业海燕出版社系列答案 本土教辅赢在暑假高效假期总复习云南科技出版社系列答案
本土教辅赢在暑假高效假期总复习云南科技出版社系列答案
相关题目
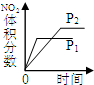
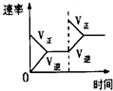
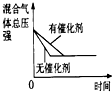
 HCO3-+H+的平衡常数K1=______.(已知:10-5.60=2.5×10-6)
HCO3-+H+的平衡常数K1=______.(已知:10-5.60=2.5×10-6) Sn(s,白)△H3=+2.1kJ?mol-1,
Sn(s,白)△H3=+2.1kJ?mol-1,

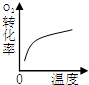
 CH3OH(g)+H2O(g),在其他条件不变的情况下,考察温度对反应的影响,实验结果如下图所示(注:T1、T2均大于300 ℃):
CH3OH(g)+H2O(g),在其他条件不变的情况下,考察温度对反应的影响,实验结果如下图所示(注:T1、T2均大于300 ℃):
 2H2O + 。
2H2O + 。