��Ŀ����
ij����M����������Ľᾧˮ����M��OH��2��xH2O��Na2CO3�Ļ���ﹲ36.8g������������ˮ�����ɰ�ɫ�����������в����ᾧˮ�������������ˡ�ϴ�ӡ���ɣ��Ƶ�������Ϊ9.85g�����õ��ij����������պ�������Ϊ7.65g����Һ�������ò��������壬�����������������Һ���ȣ������4.48L���壨��״������
��1����Һ��n��OH����=____________mol
��2��M�����ԭ������_____________________
��3��M����������Ľᾧˮ����Ļ�ѧʽΪ____________________
��4����M�Ĵ�����������NH4Cl����ͼ��ʾװ�ã�����Ƭ�����ձ��ײ�֮����һ����ˮ���л�Ϸ�Ӧ�����������ձ�ʱ������Ƭ��___________________����÷�Ӧ��Ӧ�������仯��ϵͼ��_________
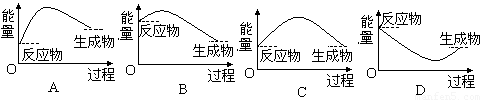
��1��0.2����2��137 ��3�� Ba��OH��2��8H2O ��4�� ���ձ�һ����������A
���������������ɵİ��������n��OH����=0.2mol/L����OH����ǰһ��û�в��뷴Ӧ����֪M��OH��2��xH2OΪ0.1mol/L������Һ�������ò���������֪M��OH��2��xH2O��Na2CO3ʱ������ȫ����Ӧ���ˡ��ֳ�����̼���Σ���MCO3 MO+CO2�������M�����ԭ������Ϊ137����M�DZ�Ԫ�ء������ɵ�9.85gBaCO3�����n��Na2CO3��=0.05mL���ٽ�ϻ���������������x=8��Ba��OH��2��8H2O��NH4Cl��Ӧʱ�����մ������ȶ�����ˮ��������ձ��벣��Ƭմ����һ�𣬸÷�Ӧ�����ȷ�Ӧ���������������ڷ�Ӧ�
MO+CO2�������M�����ԭ������Ϊ137����M�DZ�Ԫ�ء������ɵ�9.85gBaCO3�����n��Na2CO3��=0.05mL���ٽ�ϻ���������������x=8��Ba��OH��2��8H2O��NH4Cl��Ӧʱ�����մ������ȶ�����ˮ��������ձ��벣��Ƭմ����һ�𣬸÷�Ӧ�����ȷ�Ӧ���������������ڷ�Ӧ�
