��Ŀ����
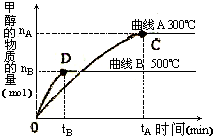 ��1�������Ϊ3L���ܱ������м���һ������CO��H2��������һ�������·�Ӧ���ɼ״���CO��g��+2H2��g����CH3OH��g������ͬ�¶������£���Ӧ�ӿ�ʼ��ƽ�⣬�״������ʵ����仯��ͼ��ʾ����÷�Ӧ��
��1�������Ϊ3L���ܱ������м���һ������CO��H2��������һ�������·�Ӧ���ɼ״���CO��g��+2H2��g����CH3OH��g������ͬ�¶������£���Ӧ�ӿ�ʼ��ƽ�⣬�״������ʵ����仯��ͼ��ʾ����÷�Ӧ����2��25���£�Mg��OH��2��s���TMg2+��aq��+2OH-��aq����Ksp=2��10-11���ڸ��¶���0.002mol?L-1MgSO4��Һ�������ҺpH����
��3�������£�ij���Na2CO3�� ��Һ�е����̪����Һ�ʺ�ɫ�������Һ��
��������1������ͼ���֪���¶�Խ�ߣ��״��ĺ���Խ�ͣ������¶�ƽ�����淴Ӧ�ƶ������ƽ���ƶ�ԭ���������
�ȸ���v=
���v��CH3OH����Ȼ���������֮�ȵ��ڻ�ѧ������֮�����v��H2����
��2������c��OH-��=
��������������Ũ�ȣ��Ӷ�ȷ����Һ��pH�����ݻ����Һ������������Ũ�������ɳ���ʱ����pH���бȽ��жϣ�
��3����̪������ɫ����֪������Һ�ʼ��ԣ����ɫ��Һ�м�������BaCl2��Һ�������̼������Һ����������ɫ�ı仯������
�ȸ���v=
| ��c |
| ��t |
��2������c��OH-��=
|
��3����̪������ɫ����֪������Һ�ʼ��ԣ����ɫ��Һ�м�������BaCl2��Һ�������̼������Һ����������ɫ�ı仯������
����⣺��1����ͼ��֪�������¶ȣ��״��ĺ������ͣ�ƽ�������ƶ��������¶�ƽ�������ȷ����ƶ����ʸ÷�Ӧ����ӦΪ���ȷ�Ӧ��
��500��ʱ��tBmin����ƽ�⣬�״���ƽ����Ӧ����v��CH3OH��=
=
mol/��L?min����������֮�ȵ��ڻ�ѧ������֮�ȣ���v��H2��=2v��CH3OH��=2��
mol/��L?min��=
mol/��L?min����
�ʴ�Ϊ�����ȣ�
mol/��L?min����
��2��0.002mol?L-1MgSO4��Һ��c��Mg2+��=0.0002mol/L������ƽ��ʱc��OH-��=
=
mol/L=10-4 mol/L����c��H+��=10-10 mol/L��������Һ��pH=-lgc��H+��=10����Ӧ������ҺpH����10��
�ʴ�Ϊ��10��
��3����̪������ɫ������Һ�ʺ�ɫ˵���Ǽ�����Һ��Ҫ��֤����Һ����̪�ʺ�ɫԭ�������·�����
����һ�����ɫ��Һ�м�������BaCl2��Һ�������Һ�Ժ�ɫ��˵������ȷ�������ɫ��ȥ��˵������ȷ��
�����������ȣ������ɫ����˵������ȷ�������ɫ���˵������ȷ��
�ʴ�Ϊ������ɫ��Һ�м�������BaCl2��Һ�������Һ���Ժ�ɫ˵������ȷ����ɫ��ȥ˵������ȷ��
��500��ʱ��tBmin����ƽ�⣬�״���ƽ����Ӧ����v��CH3OH��=
| ||
| tB min |
| nB |
| 3tB |
| nB |
| 3tB |
| 2nB |
| 3tB |
�ʴ�Ϊ�����ȣ�
| 2nB |
| 3tB |
��2��0.002mol?L-1MgSO4��Һ��c��Mg2+��=0.0002mol/L������ƽ��ʱc��OH-��=
|
|
�ʴ�Ϊ��10��
��3����̪������ɫ������Һ�ʺ�ɫ˵���Ǽ�����Һ��Ҫ��֤����Һ����̪�ʺ�ɫԭ�������·�����
����һ�����ɫ��Һ�м�������BaCl2��Һ�������Һ�Ժ�ɫ��˵������ȷ�������ɫ��ȥ��˵������ȷ��
�����������ȣ������ɫ����˵������ȷ�������ɫ���˵������ȷ��
�ʴ�Ϊ������ɫ��Һ�м�������BaCl2��Һ�������Һ���Ժ�ɫ˵������ȷ����ɫ��ȥ˵������ȷ��
���������⿼���Ϊ�ۺϣ��漰��ѧƽ��Ӱ�����ء���ѧ��Ӧ���ʡ��ܶȻ��йؼ��㡢����ˮ���֪ʶ�㣬�Ǹ߿����ȵ㣬Ӧ�������գ��Ѷ��еȣ�

��ϰ��ϵ�д�
 һ����ʦ�����Ծ�ϵ�д�
һ����ʦ�����Ծ�ϵ�д� �����Ծ���Ԫ���Ծ�ϵ�д�
�����Ծ���Ԫ���Ծ�ϵ�д�
�����Ŀ
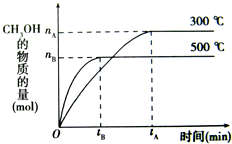 һ�������£������Ϊ3L���ܱ������л�ѧ��ӦCO��g��+2H2��g��-CH3OH��g���ﵽƽ��״̬��
һ�������£������Ϊ3L���ܱ������л�ѧ��ӦCO��g��+2H2��g��-CH3OH��g���ﵽƽ��״̬��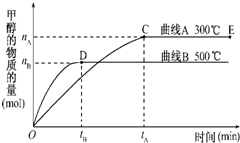 һ�������£������Ϊ3L���ܱ������У�һ����̼��������Ӧ���ɼ״�������ΪCu2O/ZnO��
һ�������£������Ϊ3L���ܱ������У�һ����̼��������Ӧ���ɼ״�������ΪCu2O/ZnO�� һ�������£������Ϊ3L���ܱ������У�һ����̼��������Ӧ���ɼ״�������ΪCu2O/ZnO����CO��g��+2H2��g��?CH3OH��g��
һ�������£������Ϊ3L���ܱ������У�һ����̼��������Ӧ���ɼ״�������ΪCu2O/ZnO����CO��g��+2H2��g��?CH3OH��g��