��Ŀ����
 һ�������£������Ϊ3L���ܱ������У�һ����̼��������Ӧ���ɼ״�������ΪCu2O/ZnO����CO��g��+2H2��g��?CH3OH��g��
һ�������£������Ϊ3L���ܱ������У�һ����̼��������Ӧ���ɼ״�������ΪCu2O/ZnO����CO��g��+2H2��g��?CH3OH��g����������������и��⣺
��1����Ӧ�ﵽƽ��ʱ��ƽ�ⳣ������ʽK=
| c(CH3OH) |
| c(CO)?c2(H2) |
| c(CH3OH) |
| c(CO)?c2(H2) |
�����¶ȣ�Kֵ
��С
��С
�����������С�����䡱������2����500�棬�ӷ�Ӧ��ʼ��ƽ�⣬������ƽ����Ӧ����v��H2��=
| 2nB |
| 3tB |
| 2nB |
| 3tB |
��3���������������������£��Դ���E�����ϵ���ѹ����ԭ����
| 1 |
| 2 |
bc
bc
a��������Ũ�ȼ��� b������Ӧ���ʼӿ죬�淴Ӧ����Ҳ�ӿ�
c���״������ʵ������� d������ƽ��ʱn��H2��/n��CH3OH������
��������1�����ݷ���ʽ��K�ĺ�����д�������¶ȶ�ƽ���Ӱ�죬�ж�K�ı仯��
��2������ͼ�м״��ı仯������������ı仯�����ٸ���v��H2��=
���㣻
��3���������������������£��Դ���E�����ϵ���ѹ����ԭ����
����ѹǿ�������淴Ӧ���ʶ�����ƽ���������ƶ����״������ʵ������࣬���������ʵ�����С�������������С��ƽ��ʱ������Ũ�ȷ���������Ũ�ȱ�ֵ�������ʵ�����ֵ��
��2������ͼ�м״��ı仯������������ı仯�����ٸ���v��H2��=
| ��c |
| t |
��3���������������������£��Դ���E�����ϵ���ѹ����ԭ����
| 1 |
| 2 |
����⣺��1����֪CO��g��+2H2��g��?CH3OH��g������K=
��500��ʱ�״������ʵ���С�����������¶ȣ�ƽ�����ƣ�����K��С��
�ʴ�Ϊ��
������
��2����500�棬ƽ��ʱͼ�м״��ı仯��ΪnB�����Է�Ӧ���ĵ���������Ϊ��2nB����v��H2��=
=
��
�ʴ�Ϊ��
��
��3���������������������£��Դ���E�����ϵ���ѹ����ԭ����
����ѹǿ�������淴Ӧ���ʶ�����ƽ���������ƶ����״������ʵ������࣬���������ʵ�����С�������������С��ƽ��ʱ������Ũ�ȷ���������Ũ�ȱ�ֵ�������ʵ�����ֵ����������ƽ��ʱ
��С����bc��ȷ��
�ʴ�Ϊ��b c��
| c(CH3OH) |
| c(CO)?c2(H2) |
�ʴ�Ϊ��
| c(CH3OH) |
| c(CO)?c2(H2) |
��2����500�棬ƽ��ʱͼ�м״��ı仯��ΪnB�����Է�Ӧ���ĵ���������Ϊ��2nB����v��H2��=
| ��c |
| t |
| 2nB |
| 3tB |
�ʴ�Ϊ��
| 2nB |
| 3tB |
��3���������������������£��Դ���E�����ϵ���ѹ����ԭ����
| 1 |
| 2 |
| c(H2) |
| c(CH3OH) |
�ʴ�Ϊ��b c��
���������⿼�黯ѧƽ���ƶ�������㣬��Ŀ�Ѷ��еȣ�����ע�����������ƽ���ƶ���Ӱ�죮

��ϰ��ϵ�д�
 ��ѧ�����ϵ�д�
��ѧ�����ϵ�д� �·Ƿ��̸����100��ϵ�д�
�·Ƿ��̸����100��ϵ�д�
�����Ŀ
 һ�������£������Ϊ3L���ܱ������У�һ����̼��������Ӧ���ɼ״�������ΪCu2O/ZnO����CO��g��+2H2��g��CH3OH��g����������������и��⣺
һ�������£������Ϊ3L���ܱ������У�һ����̼��������Ӧ���ɼ״�������ΪCu2O/ZnO����CO��g��+2H2��g��CH3OH��g����������������и��⣺ һ�������£������Ϊ3L���ܱ������У�һ����̼��������Ӧ���ɼ״�������ΪCu2O/ZnO����CO��g��+2H2��g��?CH3OH��g���������й�˵����ȷ���ǣ�������
һ�������£������Ϊ3L���ܱ������У�һ����̼��������Ӧ���ɼ״�������ΪCu2O/ZnO����CO��g��+2H2��g��?CH3OH��g���������й�˵����ȷ���ǣ�������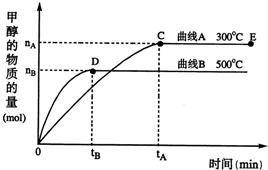 �������������ЧӦ����Դ��ȱ����������ӣ���ν��ʹ�����CO2�ĺ�������Ч�ؿ�������CO2�������˸������ձ����ӣ�Ŀǰ��ҵ����һ�ַ�������CO2������ȼ�ϼ״���һ�������£������Ϊ3L���ܱ������У�һ����̼��������Ӧ���ɼ״�������ΪCu2O/ZnO����
�������������ЧӦ����Դ��ȱ����������ӣ���ν��ʹ�����CO2�ĺ�������Ч�ؿ�������CO2�������˸������ձ����ӣ�Ŀǰ��ҵ����һ�ַ�������CO2������ȼ�ϼ״���һ�������£������Ϊ3L���ܱ������У�һ����̼��������Ӧ���ɼ״�������ΪCu2O/ZnO���� CH3OH��g��
CH3OH��g�� 2Cu+CO2
2Cu+CO2
 һ�������£������Ϊ3L���ܱ������У�һ����̼��������Ӧ���ɼ״�������ΪCu2O/ZnO������������������и��⣺
һ�������£������Ϊ3L���ܱ������У�һ����̼��������Ӧ���ɼ״�������ΪCu2O/ZnO������������������и��⣺