��Ŀ����
��ͼ�Ǵ�ͳ�Ĺ�ҵ�����������Ļ�������ͼ��
��ش�
��1�����������������������������Ʋ;߲������������ʱ�������ԡ����Ե�ʳ�д����������������Һ��Ӧ�����ӷ���ʽ��_____________________________________��
ʯ�����ƺ�ú�ĸ����Ʒ________(����������)����������������������ϡ�
��2����ұ�������У�����������Ҫ���ڽ��и�����ԭ���Ǹü����ϲ��ϱ����ģ��������������ԭ����____________________________________(�û�ѧ����ʽ��ʾ)��
��3����ҵ��ͨ��������ڵ�MgCl2��ȡ����þ����ⷴӦ����ʽΪ________________��þ�������ǻ��ý�����Ϊʲô�ڵ��ұ�������У�һ�����Ȼ��һ�����������˵�����ɡ�________________________________________________��
��1��2Al��2OH����2H2O=2AlO2-��3H2����ʯī(��̼)
��2��2C��O2 2CO
2CO
��3��MgCl2(����) Mg��Cl2����MgO���ܵ�̫�ߣ���MgCl2���۵�ϵͣ��ۻ�ʱMgCl2�ܷ�����������磻AlCl3�ǹ��ۻ�����ۻ�ʱ���ܷ�������
Mg��Cl2����MgO���ܵ�̫�ߣ���MgCl2���۵�ϵͣ��ۻ�ʱMgCl2�ܷ�����������磻AlCl3�ǹ��ۻ�����ۻ�ʱ���ܷ�������
����

�������Ļ�����Ӧ�ù㷺����FeCl3������������ӡˢ��·ͭ�帯ʴ��������ֹѪ���ȡ�
��1��д��FeCl3��Һ��ʴӡˢ��·ͭ������ӷ���ʽ ��
| |
������Ӧ ������Ӧ ��
��3����ʴͭ���Ļ����Һ�У���Cu2����Fe3����Fe2����Ũ�Ⱦ�Ϊ
0.10mol��L��1��������±����������ݺ�ҩƷ��������ȥCuCl2��Һ��Fe3����Fe2����ʵ�鲽�� ��
| | �������↑ʼ����ʱ��pH | �������������ȫʱ��pH |
| Fe3�� Fe2�� Cu2�� | 1.9 7.0 4.7 | 3.2 9.0 6.7 |
| �ṩ��ҩƷ��Cl2 ŨH2SO4 NaOH��Һ CuO Cu | ||
��4��ij������Ա�������ʲ���������и�ʴ����������ijЩ����Һ�и�ʴ�������ԡ�����ϱ��ṩ��ҩƷ��ѡ�����֣�ˮ����ѡ����������ʵ�飬��֤���ʲ�����ױ���ʴ��
�йط�Ӧ�Ļ�ѧ����ʽ
���ʲ���ָ�ʴ��ʵ������
����������������(��Ҫ�ɷ�ΪFe2O3��FeO��SiO2��)Ϊԭ���Ʊ��ߵ���������(Fe2O3)�����������������£�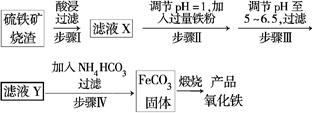
�Իش��������⣺
(1)��ҺX�к��еĽ�����������______________(�����ӷ���)��
(2)������п�ѡ��________������Һ��pH(����ĸ)��
| A��ϡ���� | B����ˮ | C������������Һ | D�����������Һ |
(4)������ķ�Ӧ�¶�һ���������35�����£���Ŀ����_____________________________________________��
(5)�ڿ���������FeCO3���ɲ�Ʒ�������Ļ�ѧ����ʽΪ______________________________��


 2MgO��2SO2����CO2��
2MgO��2SO2����CO2��