��Ŀ����
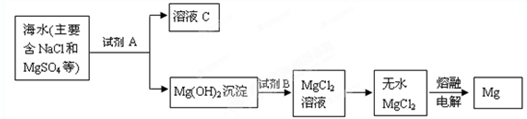
��1���Ӻ�ˮ����ȡþ����Ҫ�������£�
�������Ӻ�ˮ����ȡþ�IJ����У��Լ�A����ѡ��______���Լ�Bѡ��______��
�ڷ����Mg��OH��2�����ҺC�г�NaCl�⣬������CaCl2��Na2SO4�����ʣ�Ϊ�˻��NaCl��Һ���ڷ�������Һ�����μ��������BaCl2��Һ��Na2CO3��Һ�����ˣ�������Һ�м����������ᣮд���������BaCl2��Һ������Ӧ�����ӷ���ʽ��______��
��2����֪A��Ӧ����㷺����������һ�ֽ������ʣ�B�Ǻ�ɫ���壬C�����嵥�ʣ���һ��������������ת����ϵ��

��д��A��B�Ļ�ѧʽ��A______��B______��
��д��A��B��C�Ļ�ѧ����ʽ______��
�ʴ�Ϊ��ʯ�����Ca��OH��2��HCl��
�ڼ������BaCl2��Һ���������Ʒ�Ӧ���ɳ�������Ӧ�ķ���ʽΪBa2++SO42-=BaSO4����
�ʴ�Ϊ��Ba2++SO42-=BaSO4����
��2����֪A��Ӧ����㷺����������һ�ֽ������ʣ�������ˮ��Ӧ��ӦΪFe��B�Ǻ�ɫ���壬ӦΪFe3O4��C�����嵥�ʣ�ӦΪH2��������ӦΪ3Fe+4H2O
 Fe3O4+4H2��
Fe3O4+4H2���������Ϸ�����֪AΪFe��BΪFe3O4���ʴ�Ϊ��Fe��Fe3O4��
��Fe��H2O��Ӧ����Fe3O4��H2����Ӧ�ķ���ʽΪ3Fe+4H2O
 Fe3O4+4H2��
Fe3O4+4H2������3Fe+4H2O
 Fe3O4+4H2��
Fe3O4+4H2����������1���Ӻ�ˮ����ȡþ��Ӧ���ں�ˮ�м���������ʯ���飬����������þ���������˺�������ᣬ�����Ȼ�þ����Ũ���ᾧ������õ�������Ȼ�þ���壬������ڵ��Ȼ�þ�ɵõ�þ��
��2����֪A��Ӧ����㷺����������һ�ֽ������ʣ�������ˮ��Ӧ��ӦΪFe��B�Ǻ�ɫ���壬ӦΪFe3O4��C�����嵥�ʣ�ӦΪH2��������ӦΪ3Fe+4H2O
 Fe3O4+4H2���Դ˽����⣮
Fe3O4+4H2���Դ˽����⣮���������⿼��������ƶϺͺ�ˮ���ۺ����ã���Ŀ�Ѷ��еȣ�����ע����ݺ�ˮ���Ʊ����̣�������ʵ����ʽ��з��������ƶ�����Ӧ����㷺���������Ľ������ʿ���ΪFe��Al������ˮ��Ӧ��ӦΪFe��

�Ӻ�ˮ����ȡþ������������þ����Ҫ��Դ����������ȡþ�Ĺ������漰�ļ������ʵ��ܶȻ�����������ѧ��֪ʶ�ش����м������⣺
|
���� |
CaCO3 |
MgCO3 |
Ca(OH)2 |
Mg(OH)2 |
|
�ܶȻ� |
2.8��10�C9 |
6.8��10�C6 |
5.5��10�C6 |
1.8��10�C11 |
(1)�ڴӺ�ˮ����ȡþʱ�������õ�����(��Ҫ�ɷ���̼���)������Ϊ (��ܡ����ܡ�)��������ĥ�ɷ�ĩֱ��Ͷ�뺣ˮ�У������� ���������ֱ��Ͷ�룬Ӧ���������δ�������д����ѧ����ʽ ��
(2)ijͬѧ��ʵ����������ģ����������̣���ʵ������ʯ�ң����������ռ���棬����Ϊ (����ԡ������ԡ�)�õ�Mg(OH)2���ڼ��Լ�ʱ��������Һ���뺣ˮ�У���˼����һ�£����ڵõ��Ļ����ϵ�м���������ռ���Һ��������� (��ܡ����ܡ�)��Mg2+ת��ΪMg(OH)2������������ (�����ӷ���ʽ��ʾ)��
(3)��֪��ˮ��þ����Ũ��Ϊ1.8��10�C3mol��L�C1����Ҫʹþ���Ӳ�����������Һ��PH���ӦΪ ��
Ŀǰ����������þ����Ҫ��Դ�Ӻ�ˮ����ȡ����������ȡþ�Ĺ������漰���ļ������ʳ����µ��ܶȻ���������������ѧ��֪ʶ�ش�����ļ������⣺?
|
���� |
CaCO3 |
MgCO3 |
Ca(OH)2 |
Mg(OH)2 |
|
�ܶȻ� |
2.8��10-9 |
6.8��10-6 |
5.5��10-6 |
1.8��10-11 |
(1)�ڴӺ�ˮ����ȡþʱ�������õ����ǣ���Ҫ�ɷ���CaCO3��������Ϊ (��ܡ����ܡ�)ͨ����������ĥ�ɷ�ĩֱ��Ͷ�뺣ˮ���Ʊ���þ�ij����������� ���������ֱ��Ͷ�룬Ӧ���������δ�������д����ѧ����ʽ�� ��������һ���ո���ܡ����˿ո��������ܡ��� �˿ո�ֻ���һ�������Ļ�ѧ��Ӧ����ʽ����
��2����֪ij�غ�ˮ�е�þ���ӵ�Ũ��Ϊ1.8��10-3 mol��L-1,������Ҫʹþ���Ӳ�����������ҺpH���ӦΪ ��
��3��ʵ�����г���CaCO3��CO2�������֮һ���Ȼ�����Ӧ�ù㷺�Ļ�ѧ�Լ���������������䶳���ȡ�Ϊ�˲ⶨij�Ȼ�����Ʒ�и�Ԫ�صĺ�������������ʵ�飺
��I��ȷ��ȡ�Ȼ�����Ʒ0.2000g�������ձ��У���������6mol/L���������������ˮʹ��Ʒ��ȫ�ܽ⣬�ټ���35mL 0.25mol/L ��NH4��2C2O4��Һ��ˮԡ���ȣ�������CaC2O4�����������飬Ca2+�ѳ�����ȫ��
��II�����˲�ϴ�ӣ�I�����ó�����
��III������������10% H2SO4��Һ������������ˮ����II���г�����ȫ�ܽ⣬��Һ�����ԣ�������75�棬������μ���0.05000 mol/L KMnO4��Һ16.00mL��ǡ����ȫ��Ӧ����ش�
��֪�ζ����̷����ķ�ӦΪ2MnO4- + 5H2C2O4 + 6H+ ="=2" Mn2+ +10 CO2��+8 H2O������ƽ��
��0.05000 mol/L KMnO4��Һ����ҺӦ���� ��ѡ���ʽ����ʽ�����ζ����С�
�ڵζ��յ������Ϊ ��
�۸��Ȼ�����Ʒ�и�Ԫ�ص������ٷ���Ϊ ��