��Ŀ����
��֪X��Y��Z��W����Ԫ����Ԫ�����ڱ�������������ͬ�����ڵ�Ԫ�أ���ԭ��������������X��Wͬ���壬Y��ZΪͬ���ڵ�����Ԫ�أ�Wԭ�ӵ�����������Y��Zԭ������������֮�ͣ�Y���⻯���������3�����ۼ���Zԭ�������������Ǵ�����������3�����ش��������⣮
��1��X��Z����Ԫ�صķ��ţ�X
��2������Ԫ�����γɵļ���������ĵ���ʽΪ��
 ��
��
��3����X��Y��Z���γɵij������ӻ�������W������������ˮ�����Ũ��Һ����ʱ��Ӧ�����ӷ���ʽ�ǣ�
��4������X��Y��Z��W����Ԫ���γɵij������ʻ����У������γɵľ���������
��1��X��Z����Ԫ�صķ��ţ�X
H
H
��ZO
O
��2������Ԫ�����γɵļ���������ĵ���ʽΪ��


��3����X��Y��Z���γɵij������ӻ�������W������������ˮ�����Ũ��Һ����ʱ��Ӧ�����ӷ���ʽ�ǣ�
NH4++OH-
NH3��+H2O
| ||
NH4++OH-
NH3��+H2O
��
| ||
��4������X��Y��Z��W����Ԫ���γɵij������ʻ����У������γɵľ���������
ԭ�Ӿ���
ԭ�Ӿ���
��������Y���⻯���������3�����ۼ���YӦΪNԪ�أ�Zԭ�������������Ǵ�����������3������ZΪOԪ�أ�Wԭ�ӵ�����������Y��Zԭ������������֮�ͣ���WΪNaԪ�أ�X��Wͬ���壬��ԭ��������С��ΪHԪ�أ���϶�Ӧ���ʺͻ���������ʽ����⣮
����⣺Y���⻯���������3�����ۼ���YӦΪNԪ�أ�Zԭ�������������Ǵ�����������3������ZΪOԪ�أ�Wԭ�ӵ�����������Y��Zԭ������������֮�ͣ���WΪNaԪ�أ�X��Wͬ���壬��ԭ��������С��ΪHԪ�أ�
��1�������Ϸ�����֪XΪH��ZΪO���ʴ�Ϊ��H��O��
��2������Ԫ�����γɵļ���������ΪNa2O������ʽΪ ���ʴ�Ϊ��
���ʴ�Ϊ�� ��
��
��3����X��Y��Z���γɵij������ӻ�����ΪNH4NO3��W������������ˮ����ΪNaOH�����߷�Ӧ�����ӷ���ʽΪNH4++OH-
NH3��+H2O��
�ʴ�Ϊ��NH4++OH-
NH3��+H2O��
��4��X��Y��Z��W����Ԫ���γɵij������ʻ����п��γɷ��Ӿ��壬��ˮ�����Ӿ�����NaOH�����������Na���������γ�ԭ�Ӿ��壬
�ʴ�Ϊ��ԭ�Ӿ��壮
��1�������Ϸ�����֪XΪH��ZΪO���ʴ�Ϊ��H��O��
��2������Ԫ�����γɵļ���������ΪNa2O������ʽΪ
 ���ʴ�Ϊ��
���ʴ�Ϊ�� ��
����3����X��Y��Z���γɵij������ӻ�����ΪNH4NO3��W������������ˮ����ΪNaOH�����߷�Ӧ�����ӷ���ʽΪNH4++OH-
| ||
�ʴ�Ϊ��NH4++OH-
| ||
��4��X��Y��Z��W����Ԫ���γɵij������ʻ����п��γɷ��Ӿ��壬��ˮ�����Ӿ�����NaOH�����������Na���������γ�ԭ�Ӿ��壬
�ʴ�Ϊ��ԭ�Ӿ��壮
���������⿼��Ԫ���ƶ��⣬�ۺϿ���ѧ�������������������Ŀ�Ѷ��еȣ�����ע�����ʽ����д�Լ�������ʵ����ʣ�Ϊ�״��㣮

��ϰ��ϵ�д�
 ��������ϵ�д�
��������ϵ�д�
�����Ŀ








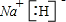
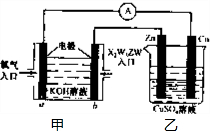 ��֪X��Y��Z��W���ֶ�����Ԫ�ص�ԭ�Ӱ뾶���μ�С�������ڱ���X��Y��Y��Z��λͬһ���ڵ�����λ�ã�X������������Ϊ������������2����W�ֱ�����X��Y��Z��һ��ԭ�������γɵ�������Ϊ10�ij�����������ж�X��Y��Z��W����Ԫ�ز��ش��������⣺��Ҫ����ȷ�����Ԫ�ط��ż��йػ�ѧ�����ʾ��
��֪X��Y��Z��W���ֶ�����Ԫ�ص�ԭ�Ӱ뾶���μ�С�������ڱ���X��Y��Y��Z��λͬһ���ڵ�����λ�ã�X������������Ϊ������������2����W�ֱ�����X��Y��Z��һ��ԭ�������γɵ�������Ϊ10�ij�����������ж�X��Y��Z��W����Ԫ�ز��ش��������⣺��Ҫ����ȷ�����Ԫ�ط��ż��йػ�ѧ�����ʾ��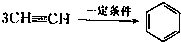 �����谷Ҳ�������谷��WXY�����е�Wԭ�ӱ�����ȡ����ɵ��谷��������������Ȳ�����۷�Ӧ���õ��������谷���Ǽ��Լ�������������������д�������谷�ṹ��ʽ��
�����谷Ҳ�������谷��WXY�����е�Wԭ�ӱ�����ȡ����ɵ��谷��������������Ȳ�����۷�Ӧ���õ��������谷���Ǽ��Լ�������������������д�������谷�ṹ��ʽ��