��Ŀ����
��֪X��Y��Z��W����Ԫ�طֱ���Ԫ�����ڱ����������������ڵ�Ԫ�أ���ԭ��������������X��Wͬ���壬Y��ZΪͬ���ڵ�����Ԫ�أ�Wԭ�ӵ�����������Y��Zԭ������������֮�ͣ�Y���⻯���������3�����ۼ������ƶϣ�
��1��X��Z����Ԫ�ص�Ԫ�ط��ţ�X
��2��������Ԫ���������γɵĻ������У�����ˮ�Լ��Ե���̬�⻯��ĵ���ʽΪ
 �����Ĺ��ۼ�����
�����Ĺ��ۼ�����
 ��
��
��3����X��Y��Z���γɵij������ӻ�������
 �����ֻ������Ũ��Һ��Ϲ��ȵ����ӷ�Ӧ����ʽΪ
�����ֻ������Ũ��Һ��Ϲ��ȵ����ӷ�Ӧ����ʽΪ
��4��X��W�γɵĻ�����ĵ���ʽΪ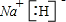
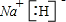 �����û���������ˮ��������Ӧ�Ļ�ѧ����ʽΪ
�����û���������ˮ��������Ӧ�Ļ�ѧ����ʽΪ
��1��X��Z����Ԫ�ص�Ԫ�ط��ţ�X
H
H
��ZO
O
����2��������Ԫ���������γɵĻ������У�����ˮ�Լ��Ե���̬�⻯��ĵ���ʽΪ


����
����
������ԡ��Ǽ��ԡ��������������Ӽ��ͷǼ��Թ��ۼ��Ļ�����ĵ���ʽΪ

��3����X��Y��Z���γɵij������ӻ�������
NH4NO3
NH4NO3
��д��ѧʽ������X��Z��W��Ԫ���γɵ����ӻ�����ĵ���ʽΪ

NH4++OH-?NH3��+H2O
NH4++OH-?NH3��+H2O
����4��X��W�γɵĻ�����ĵ���ʽΪ
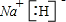
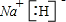
NaH+H2O�TNaOH+H2��
NaH+H2O�TNaOH+H2��
������ˮ������
����
�������������ԭ������������X��Y��Z��W����Ԫ�طֱ���Ԫ�����ڱ����������������ڵ�Ԫ�أ���ԭ����������������XΪHԪ�أ�Y���⻯���������3�����ۼ���˵������������Ϊ5��Y���ڢ�A�壬X��Wͬ���壬���ڢ�A�壬Y��ԭ������С��W��������Ԫ�أ���YΪNԪ�ء�WΪNaԪ�أ�Y��ZΪͬ���ڵ�����Ԫ�أ�Wԭ�ӵ�����������Y��Zԭ������������֮�ͣ�Z��ԭ������������Ϊ11-5=6��ԭ������С��NaԪ�ء�����NԪ�أ���ZΪOԪ�أ��ݴ˽��
����⣺X��Y��Z��W����Ԫ�طֱ���Ԫ�����ڱ����������������ڵ�Ԫ�أ���ԭ����������������XΪHԪ�أ�Y���⻯���������3�����ۼ���˵������������Ϊ5��Y���ڢ�A�壬X��Wͬ���壬���ڢ�A�壬Y��ԭ������С��W��������Ԫ�أ���YΪNԪ�ء�WΪNaԪ�أ�Y��ZΪͬ���ڵ�����Ԫ�أ�Wԭ�ӵ�����������Y��Zԭ������������֮�ͣ�Z��ԭ������������Ϊ11-5=6��ԭ������С��NaԪ�ء�����NԪ�أ���ZΪOԪ�أ�
��1��������������֪��XΪHԪ�ء�ZΪOԪ�أ�
�ʴ�Ϊ��H��O��
��2��������Ԫ���������γɵĻ������У�����ˮ�Լ��Ե���̬�⻯��ΪNH3�������ʽΪ �����Ĺ��ۼ����ڼ��Լ������������Ӽ��ͷǼ��Թ��ۼ��Ļ�����Na2O2�������ʽΪ
�����Ĺ��ۼ����ڼ��Լ������������Ӽ��ͷǼ��Թ��ۼ��Ļ�����Na2O2�������ʽΪ ��
��
�ʴ�Ϊ�� �����ԣ�
�����ԣ� ��
��
��3����H��N��O���γɵij������ӻ�������NH4NO3����H��O��Na��Ԫ���γɵ����ӻ�����ΪNaOH�������ʽΪ �����ֻ������Ũ��Һ��Ϲ��ȵ����ӷ�Ӧ����ʽΪ NH4++OH-?NH3��+H2O��
�����ֻ������Ũ��Һ��Ϲ��ȵ����ӷ�Ӧ����ʽΪ NH4++OH-?NH3��+H2O��
�ʴ�Ϊ��NH4NO3�� ��NH4++OH-?NH3��+H2O��
��NH4++OH-?NH3��+H2O��
��4��H��Na�γɵĻ�����ΪNaH���������ӻ��������ʽΪ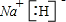 �����û���������ˮ��������Ӧ����������������������Ӧ��ѧ����ʽΪNaH+H2O�TNaOH+H2��������ˮ����Ԫ�ػ��ϼ۽��ͣ�ˮ����������
�����û���������ˮ��������Ӧ����������������������Ӧ��ѧ����ʽΪNaH+H2O�TNaOH+H2��������ˮ����Ԫ�ػ��ϼ۽��ͣ�ˮ����������
�ʴ�Ϊ��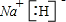 ��NaH+H2O�TNaOH+H2����������
��NaH+H2O�TNaOH+H2����������
��1��������������֪��XΪHԪ�ء�ZΪOԪ�أ�
�ʴ�Ϊ��H��O��
��2��������Ԫ���������γɵĻ������У�����ˮ�Լ��Ե���̬�⻯��ΪNH3�������ʽΪ
 �����Ĺ��ۼ����ڼ��Լ������������Ӽ��ͷǼ��Թ��ۼ��Ļ�����Na2O2�������ʽΪ
�����Ĺ��ۼ����ڼ��Լ������������Ӽ��ͷǼ��Թ��ۼ��Ļ�����Na2O2�������ʽΪ ��
���ʴ�Ϊ��
 �����ԣ�
�����ԣ� ��
����3����H��N��O���γɵij������ӻ�������NH4NO3����H��O��Na��Ԫ���γɵ����ӻ�����ΪNaOH�������ʽΪ
 �����ֻ������Ũ��Һ��Ϲ��ȵ����ӷ�Ӧ����ʽΪ NH4++OH-?NH3��+H2O��
�����ֻ������Ũ��Һ��Ϲ��ȵ����ӷ�Ӧ����ʽΪ NH4++OH-?NH3��+H2O���ʴ�Ϊ��NH4NO3��
 ��NH4++OH-?NH3��+H2O��
��NH4++OH-?NH3��+H2O����4��H��Na�γɵĻ�����ΪNaH���������ӻ��������ʽΪ
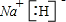 �����û���������ˮ��������Ӧ����������������������Ӧ��ѧ����ʽΪNaH+H2O�TNaOH+H2��������ˮ����Ԫ�ػ��ϼ۽��ͣ�ˮ����������
�����û���������ˮ��������Ӧ����������������������Ӧ��ѧ����ʽΪNaH+H2O�TNaOH+H2��������ˮ����Ԫ�ػ��ϼ۽��ͣ�ˮ�����������ʴ�Ϊ��
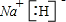 ��NaH+H2O�TNaOH+H2����������
��NaH+H2O�TNaOH+H2�������������������⿼��ṹ����λ�ù�ϵ�����û�ѧ����Ѷ��еȣ�ע�����ճ��ó��û�ѧ�������д����4���и���ˮ��ԭ����д����ʽ��

��ϰ��ϵ�д�
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
�����Ŀ





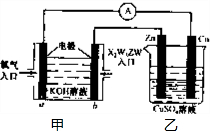 ��֪X��Y��Z��W���ֶ�����Ԫ�ص�ԭ�Ӱ뾶���μ�С�������ڱ���X��Y��Y��Z��λͬһ���ڵ�����λ�ã�X������������Ϊ������������2����W�ֱ�����X��Y��Z��һ��ԭ�������γɵ�������Ϊ10�ij�����������ж�X��Y��Z��W����Ԫ�ز��ش��������⣺��Ҫ����ȷ�����Ԫ�ط��ż��йػ�ѧ�����ʾ��
��֪X��Y��Z��W���ֶ�����Ԫ�ص�ԭ�Ӱ뾶���μ�С�������ڱ���X��Y��Y��Z��λͬһ���ڵ�����λ�ã�X������������Ϊ������������2����W�ֱ�����X��Y��Z��һ��ԭ�������γɵ�������Ϊ10�ij�����������ж�X��Y��Z��W����Ԫ�ز��ش��������⣺��Ҫ����ȷ�����Ԫ�ط��ż��йػ�ѧ�����ʾ��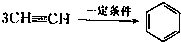 �����谷Ҳ�������谷��WXY�����е�Wԭ�ӱ�����ȡ����ɵ��谷��������������Ȳ�����۷�Ӧ���õ��������谷���Ǽ��Լ�������������������д�������谷�ṹ��ʽ��
�����谷Ҳ�������谷��WXY�����е�Wԭ�ӱ�����ȡ����ɵ��谷��������������Ȳ�����۷�Ӧ���õ��������谷���Ǽ��Լ�������������������д�������谷�ṹ��ʽ��