��Ŀ����
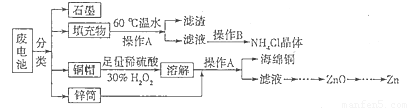
�Ͼɵ�ؽ��뻷�����������һϵ�е��»����°����±��Σ�����ϵ������Ҫ��ͭñ����Cu��Zn����п�ǡ���Ƭ��ʯī������MnO2��NH4Cl�����ԷϾɵ�ؽ�����Դ�������Ĺ����������£�

��1������A������Ϊ �������ijɷ�Ϊ ��
��2���������60����ˮ�ܽ⣬Ŀ����Ϊ�˼ӿ��ܽ����ʣ�����������¶Ȳ���̫�ߣ���ԭ���� ��
��3��ͭñ�ܽ�ʱ����H2O2��Ŀ���ǣ��û�ѧ����ʽ��ʾ�� ��ͭñ�ܽ���ȫ���轫��Һ�й�����H2O2��ȥ����ȥH2O2�ļ�㷽���� ��
��4������п�̵�صĵ����ΪKOH���ܷ�ӦΪ��Zn+2MnO2+2H2O===2MnOOH+Zn(OH)2���为���ĵ缫��ӦʽΪ ��
��14�֣���1�����ˣ�2�֣� MnO2��2�֣�
��2����ֹ������NH4Cl�ֽ⣨2�֣�������������Ҳ���֣�
��3����Cu+H2O2+H2SO4==CuSO4+2H2O��3�֣�����ƽ��1�֣���ѧʽ�������֣�
�ڼ��ȣ�2�֣�
��4��Zn��2e��+2OH��==Zn(OH)2��3�֣�
��������
�����������1�����ݻ��������ᴿ�ķ�����֪���õ���������Һ�ķ����ǹ��ˣ������A������Ϊ���˻���ȹ��ˣ���60����ˮ�ܽ�MnO2��NH4Cl�Ļ����õ���������MnO2��Ϊ�˷�ֹ��ȴʱ�ᾧ����NH4Cl�����������ˮ�����������ó��ȹ��ˣ���2�����ȣ�NH4Cl��ǿ�������Σ��¶�̫�ߴٽ��䳹��ˮ�⣬��Ϊ�����ֽ��NH3•H2O���ӷ���HCl������NH4Cl�IJ��ʣ���Σ��¶�̫�ߣ�NH4Cl�����ֽ�ΪNH3��HCl���壻��3��Cu��������������ֱ������ϡ���ᣬ���������dz����������������������������ܽ�ͭ���ʣ����ݻ��ϼ�����������ȡ�ͭ�����ԭ���غ�ɵã�Cu+H2O2+H2SO4==CuSO4+2H2O�������Լ�������������Լ����ȥ��˫��ˮ�������ֽ⣬����ͭ���Դ���ֽ⣬��˼����dz�ȥ��������ļ�㷽���������������п�ң�Zn�����Գ�ȥCu2+��������ϡ���ᣬ���˵õ�������Ϊ����ͭ��Cu������ҺΪ����п��Һ������ҺΪԭ�Ͽ����Ʊ�ZnO����4�������ܷ�Ӧʽ�л��ϼ����ߵ�Ԫ�ؿ�֪����������Ҫ��Ӧ�������Ϊ��Zn��Zn(OH)2���ɻ��ϼ�������������ʧȥ�������ɵã�Zn��2e����Zn(OH)2�����ݵ���غ��ԭ���غ��֪��������ӦʽΪZn��2e��+2OH��==Zn(OH)2��
���㣺����Ͼɵ�ش����Ļ��������⣬�漰��ѧʵ�������������ε���Ҫ���ʡ�ͭ����Ҫ���ʡ������������Ҫ���ʡ�������Ӧʽ��֪ʶ��