��Ŀ����
����Ŀ����һ��������Fe��Fe2O3��CuO�Ļ�Ϸ�ĩ���뵽100mL 4.4mol/L �������У���ַ�Ӧ��ַ�Ӧ�����ɱ�״���µ�����896mL�����ˣ�������ϴ�ӡ���������������Ϊ1.28g����Һ�е�����ֻ��FeCl2��HCl����Һ��ˮϡ�͵�320mLʱ�������Ũ��Ϊ0.25mol/L��
��ش�
(1)��Ӧ�������������ʵ���Ϊ_______ mol��
(2)����ԭ������������ʵ�����(д���������)��________
���𰸡�0.04 5.6g
��������
(1)����n=![]() �����������������ʵ�����
�����������������ʵ�����
(2)����������ᷴӦ��������ʣ�࣬�������û��Fe��ֻ��Cu����Һ�к���ʣ���HCl��FeCl2������Clԭ���غ�n��(HCl)=nʣ��(HCl)+2n(FeCl2)���ݴ˼���n(FeCl2)����ԭ��FeΪx mol��Fe2O3Ϊy mol������FeԪ���غ�͵���ת���غ��з��̼�����
(1)���ɱ�״���µ�����896mL�������ʵ���Ϊ![]() =0.04mol���ʴ�Ϊ��0.04��
=0.04mol���ʴ�Ϊ��0.04��
(2)����������ᷴӦ��������ʣ�࣬�������û��Fe��ֻ��Cu����Һ�к���ʣ���HCl��FeCl2������Clԭ���غ㣺n��(HCl)=nʣ��(HCl)+2n(FeCl2)����0.1L��4.4mol/L=0.32L��0.25mol/L+2n(FeCl2)�����n(FeCl2)=0.18mol��n(CuO)=n(Cu)=![]() =0.02mol����ԭ��FeΪx mol��Fe2O3Ϊy mol����FeԪ���غ㣬�ɵã�x+2y=0.18�����ݵ���ת���غ㣬�ɵã�2x=2y+2��0.02+2��0.04���������̣���ã�x=0.1��y=0.04��ԭ������е�����������56g/mol��0.1mol=5.6g���ʴ�Ϊ��5.6g��
=0.02mol����ԭ��FeΪx mol��Fe2O3Ϊy mol����FeԪ���غ㣬�ɵã�x+2y=0.18�����ݵ���ת���غ㣬�ɵã�2x=2y+2��0.02+2��0.04���������̣���ã�x=0.1��y=0.04��ԭ������е�����������56g/mol��0.1mol=5.6g���ʴ�Ϊ��5.6g��

����Ŀ����1����0.3mol����̬����ȼ�������飨B2H6����������ȼ�գ����ɹ�̬�����������Һ̬ˮ���ų�649.5kJ�������÷�Ӧ���Ȼ�ѧ����ʽΪ______________������֪��H2O��g��=H2O��l������H2����44.0kJ/mol����11.2L����״������������ȫȼ��������̬ˮʱ�ų���������_____________kJ��
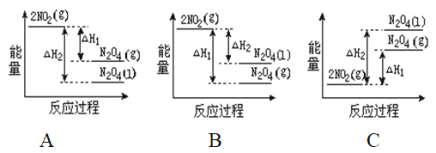
��2����֪��2NO2��g��![]() N2O4��g����H1 2NO2��g��
N2O4��g����H1 2NO2��g��![]() N2O4��l����H2
N2O4��l����H2
���������仯ʾ��ͼ�У���ȷ���ǣ�ѡ����ĸ��_____________��
��3�����ݸ�˹���ɿ��Զ�ijЩ����ͨ��ʵ��ֱ�Ӳⶨ�Ļ�ѧ��Ӧ���ʱ�������㡣
��֪��C��s��ʯī����O2��g����CO2��g�� ��H1����393.5 kJ��mol��1
2H2��g����O2��g����2H2O��l�� ��H2����571.6 kJ��mol��1
2C2H2��g����5O2��g����4CO2��g����2H2O��l�� ��H3����2 599 kJ��mol��1
���ݸ�˹���ɣ�����298 Kʱ��C��s��ʯī����H2��g������1 mol C2H2��g����Ӧ���ʱ䣨�г��ļ���ʽ����___________________________��
��4���״���һ�����͵���������ȼ�ϣ���ҵ�Ͽ�ͨ��CO��H2�������Ʊ��״����壨�ṹ��ʽΪCH3OH���� ��֪ijЩ��ѧ���ļ����������±���
��ѧ�� | C��C | C��H | H��H | C��O | C��O | H��O |
����/kJ��mol��1 | 348 | 413 | 436 | 358 | 1072 | 463 |
��֪CO�е�C��O֮��Ϊ�������ӣ���ҵ�Ʊ��״����Ȼ�ѧ����ʽΪ_________��
����Ŀ���±���Ԫ�����ڱ���һ���֣���Ա��еĢ١�����Ԫ�أ��밴Ҫ����д���пհף�
���� ���� | ��A | ��A | ��A | ��A | ��A | ��A | ��A |
2 | �� | �� | �� | �� | |||
3 | �� | �� | �� | �� |
(1)��Ԫ�آ٢ڢݢޢߢ��Ӧ������������ˮ�����У�������ǿ�Ļ����������ʽ�ǣ�_________��
(2)д��Ԫ�آڵ�����⻯��Ļ�ѧʽ____________��
(3)�ܢݢޢ�����Ԫ�صļ����Ӱ뾶�Ӵ�С����____________(�����ӷ��ű�ʾ)��
(4)д��Ԫ�آ������������Ԫ�آݵ�����������ˮ���ﷴӦ�����ӷ���ʽ______________________________________��
(5)д��Ԫ�آ۵ij����⻯�����������������ˮ���ﷴӦ�Ļ�ѧ����ʽ_______________��