��Ŀ����
��14�֣�������Ԫ���γɵij����ǽ������嵥��A�볣����������B���ڼ��������·�Ӧ���ɻ�����C��C��ˮ��Ӧ���ɰ�ɫ����D����ɫ���г�������ζ������E��D��������ǿ�ᣬҲ������ǿ�E���Ӻ�18�����ӣ�������������ȼ������G��G�ڴ������ܵ���������γɡ�E����������������Һ���յõ���ɫ��ҺF����ҺF�ڿ����г��ڷ��÷�����Ӧ��������֮һΪH��H��������ƵĽṹ�ͻ�ѧ�������ƣ�����Һ�Ի�ɫ��
��1����ɵ���A��Ԫ��λ�����ڱ��е� ���ڵ� �壻
��2��������H�ĵ���ʽΪ ��
��3��B������������Һ��Ӧ�����ӷ���ʽΪ�� ��
��4��G����������Һ��Ӧ����������ɱ�����������ȡ�д����Ӧ�����ӷ���ʽ��
��5����ҺF�ڿ����г��ڷ�������H�Ļ�ѧ��Ӧ����ʽΪ�� ��
��6��д��F��Һ�и�����Ũ���ɴ�С�Ĺ�ϵΪ�� ��
��14�֣�ÿ��2�֣���1���� �� ��A ��2��
��3��2Al��2H2O��2OH��=2AlO2����3H2�� ��4��SO2��2ClO3��=SO42����2ClO2
��5��4Na2S��O2��2H2O=4NaOH��2Na2S2
����2Na2S��O2��2H2O=4NaOH��2S �� Na2S��S=Na2S2 ��
��6��c��Na���� > c��S2���� > c��OH���� > c��HS���� > c��H����
����:��

 ��У����ϵ�д�
��У����ϵ�д�

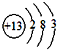


 ��������ʱ��ת�Ƶ���
��������ʱ��ת�Ƶ���