��Ŀ����
��֪����������ˮ�д��������ܽ�ƽ�⣺Ca(OH)2(s) �PCa2++2OH-���ڻ������糧ȼ��ú�ķ������������ж�������������������������̼�ȡ�Ϊ�˳�ȥ�к�������������Ϊ���������÷�ĩ״��̼��ƻ���ʯ�ҵ�����Һϴ�ӷ�������Ӧ����Ϊʯ��[CaSO4��2H2O]��
(1)д������������Ӧ�Ļ�ѧ����ʽ����_____________����_________________��
��2����˵������ʯ�ҵ�����Һ�������ó���ʯ��ˮϴ�ӷ��������ɣ�________��
��3����Ӣ�����е�һ���о�������������̴ѿ�����Ч�ؽ��͵ر�����������Ũ�ȡ��ڶ�ʮ��������ʮ�����10��䣬�ɷ��糧�ŷų��Ķ�������������35%�������ڽ�����̴ѵĽ��ʹ������������Ũ�Ƚ�����30%֮�ࡣ���ȫ�������ĽǶȣ��������ַ����Ƿ��ȡ����____________________��
��1����2SO2+O2+2CaCO3=2CaSO4+2CO2
��2SO2+O2+2CaCO3+4H2O=2[CaSO4��2H2O]+2CO2
�� 2SO2+O2+2Ca(OH)2=2CaSO4+2H2O
��2SO2+O2+2Ca(OH)2+2H2O=2[CaSO4��2H2O]
��2��������������ˮ�������ʯ��ˮ���������Ƶ�Ũ��С�������ڶ�����������ա�
��3������ȡ����Ϊ����������ŷ�����û�м��٣���һ���γ������Ի���ɶ�ȫ����Σ����
����:
��1����2SO2+O2+2CaCO3=2CaSO4+2CO2
��2SO2+O2+2CaCO3+4H2O=2[CaSO4��2H2O]+2CO2
�� 2SO2+O2+2Ca(OH)2=2CaSO4+2H2O
��2SO2+O2+2Ca(OH)2+2H2O=2[CaSO4��2H2O]
��2��������������ˮ�������ʯ��ˮ���������Ƶ�Ũ��С�������ڶ�����������ա�
��3������ȡ����Ϊ����������ŷ�����û�м��٣���һ���γ������Ի���ɶ�ȫ����Σ����
��ע����ҵ�ϴ���β����������ķ����ж��֣���ѧϰ��һ֪ʶʱҪע�ⲻҪ˼ά���ƣ�Ҫѧ�����˼�����Ӷ���������������������������Ժͻ�ԭ�ԵȽǶ�ȥ���⡣

 ��
�� ��
��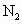 ��
�� �ȣ�Ϊ�˳�ȥ�к�����
�ȣ�Ϊ�˳�ȥ�к����� ����Һ��Ӧ��________
����Һ��Ӧ��________ ����Һ��Ӧ��________
����Һ��Ӧ��________